-
iestinstrument
Oxide Solid Electrolyte Evaluation: High-Throughput Ionic Conductivity & Semi-Solid Battery Testing Solutions
1. Abstract
Oxide solid electrolytes are a leading candidate for high-energy, high-safety next-generation batteries. This article presents IEST’s practical solutions for rapid, reliable oxide solid electrolyte characterization — from pellet fabrication and high-pressure densification to high-frequency impedance measurement and semi-solid battery testing. We describe the SEMS1100 testing platform, a hot-pressing rapid densification workflow, and best practices to obtain ionic conductivity data that are more predictive of cell-level performance. The goal is to accelerate material screening while improving the correlation between lab measurements and real battery behavior.
At IEST, we provide a comprehensive suite of cutting-edge testing solutions designed specifically for solid-state batteries—from material-level characterization to full-cell performance evaluation, from multi-dimensional powder compression & EIS (SEMS series) to glovebox-integrated single-particle mechanics, automatic mold-pressing, SWE in-situ swelling analyzers and BPD pressure/temperature mapping. Our integrated systems enable researchers and manufacturers to accurately measure mechanical, electrochemical, and thermal behaviors under realistic operating conditions, accelerating the development and commercialization of next-generation batteries.
Figure 1. Comprehensive solutions for solid-State Battery Testing
2. Background — why oxide solid electrolytes matter
Current lithium-ion batteries are classified based on liquid electrolyte content. Semi-solid batteries (5-10 wt% liquid electrolyte) and all-solid-state batteries (0 wt% liquid electrolyte) are collectively termed solid-state batteries. Semi-solid-state batteries reduce liquid electrolyte usage by incorporating composite electrolytes of oxides and polymers. Oxides are primarily applied as coatings on separators or electrode surfaces, while polymers form framework networks. Additionally, anodes have evolved from graphite to pre-lithiated silicon-based or lithium metal anodes, cathodes transition from high-nickel to high-voltage platforms (e.g., high-nickel, lithium-rich manganese-based cathodes), and separators retain solid electrolyte coatings. Lithium salts such as LiPF6 are being replaced by LiTFSI, achieving energy densities exceeding 350 Wh/kg. However, flammability risks persist in semi-solid-state systems due to residual liquid electrolytes. In contrast, all-solid-state batteries eliminate liquid electrolytes entirely, employing oxide-, sulfide-, halide-, or polymer-based solid electrolytes as thin-film separators. Oxides currently lead in commercialization progress, while sulfides show the greatest future potential. Anodes and cathodes follow similar upgrade paths as semi-solid systems, with energy densities surpassing 500 Wh/kg. As summarized in the 2022 roadmap “Toward Better Batteries: Solid-State Battery Roadmap 2035+“, numerous component combinations (e.g., anode/cathode active materials, anode/cathode electrolytes, and SE separators) have been explored. Recent breakthroughs highlight oxide-based solid-state electrolytes as one of the earliest developed and most studied materials.
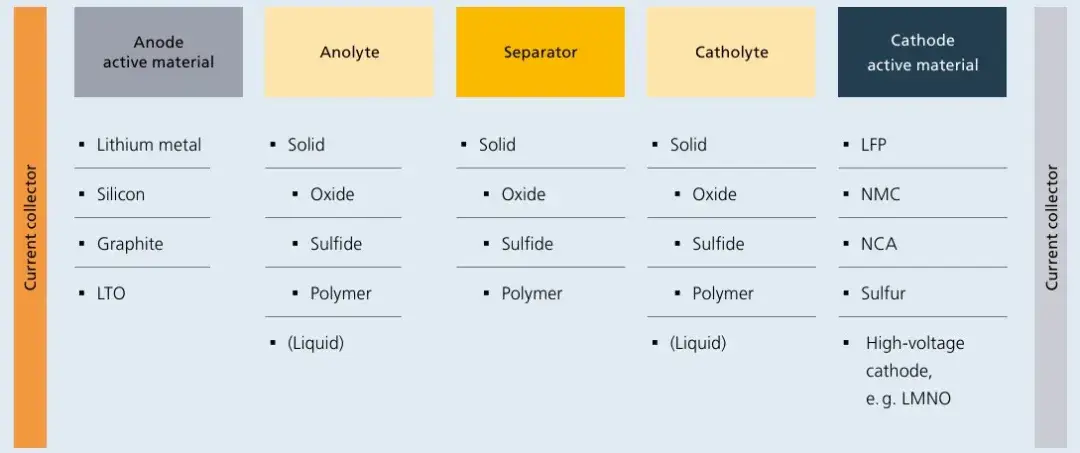
Figure 2. Composition of solid-state battery components
Accurate ionic conductivity measurement is essential for assessing oxide electrolytes because ion transport and interfacial contact dominate solid-state cell performance. However, conventional sintering, metallization and pellet preparation are time-consuming and not always representative of compacted powder or coated applications. This creates a bottleneck for high-throughput materials discovery and for testing semi-solid electrode architectures that use oxide coatings or powder composites.
3. Key challenges in oxide electrolyte testing
-
High densification requirement: Oxide conductors typically need >95% densification (thick, sintered pellets >500 μm) to reach intrinsic bulk conductivity.
-
Contact reliability: Metallized contacts (Au/Pt/Ag/C sputtered layers) and edge insulation are necessary to prevent electronic shorting but add process overhead.
-
Thermal and mechanical effects: Measurements are pressure- and temperature-dependent; interface resistance with electrodes or current collectors can dominate EIS results.
-
Throughput gap: Traditional sintering and metallization pipelines are labor-intensive, limiting rapid screening of novel compositions and processing conditions.
-
Application relevance: Powder-pressed or coated oxide layers used in semi-solid battery designs require conductivity evaluation under realistic compaction and thermal conditions.
4. IEST SEMS1100 testing platform — an integrated solution
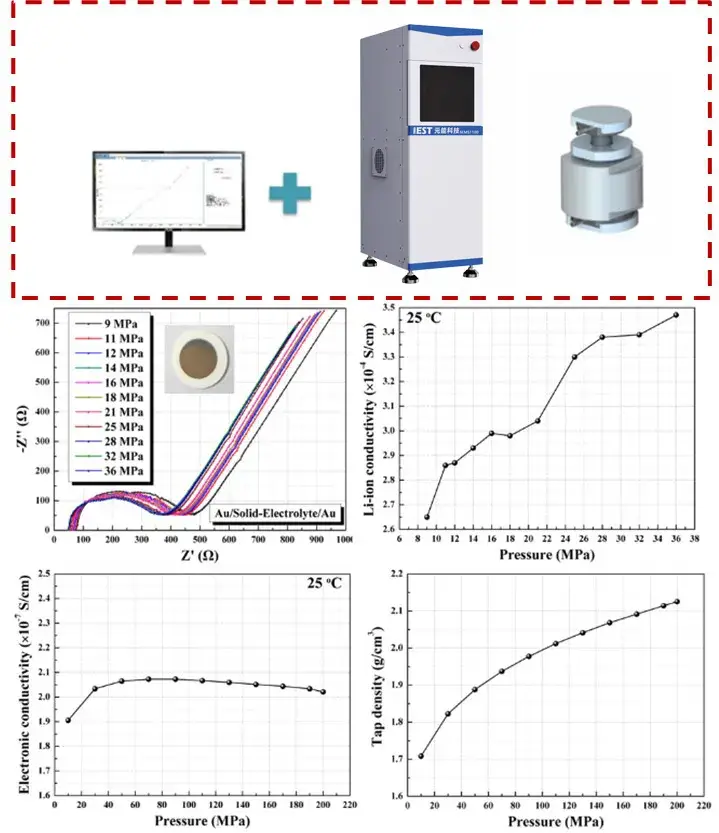
IEST’s SEMS1100 series was designed to address these bottlenecks by combining pellet preparation, controlled pressure application, high-frequency impedance measurement (>10 MHz capability) and auxiliary diagnostics into one workflow. Key features:
-
Stable pellet pressing: Precision press capable of applying controlled, reproducible pressures to powder or partially processed pellets.
-
High-frequency EIS: Broadband impedance to separate bulk, grain-boundary and interfacial contributions.
-
Integrated electronic conductivity testing: Parallel measurement of electronic leakage to confirm ionic-dominated transport.
-
Temperature & pressure control: Test conditions from ambient to elevated temperatures under defined mechanical loads to replicate stack compression.
-
Data pipeline: Automated fitting routines produce ionic conductivity, activation energy, and compaction-dependent trends for rapid comparison.
SEMS1100 enables researchers to quantify how pressure, temperature and post-processing influence measured ionic conductivity, improving comparability across labs and with cell-level tests.
Figure 3. SEMS1100 solid electrolyte ionic conductivity test system and application scenarios
5. Rapid hot-pressing workflow for powder screening
To accelerate screening while preserving measurement relevance, IEST and academic partners developed a high-temperature hot-pressing workflow:
-
Direct powder hot-press: Apply synchronized pressure and moderate temperatures (e.g., 300–500 °C) to compact powders rapidly without full conventional sintering.
-
Post-press metallization: Apply conductive contacts (silver paste or thin metal layers) after pressing to enable EIS while avoiding high-temperature sputtering steps.
-
High-pressure EIS: Measure impedance under controlled contact pressure to reduce interfacial artifacts.
-
Correlative validation: Benchmark hot-pressed pellet conductivity against conventionally sintered reference pellets to calibrate transfer functions.
This approach reduces turnaround from days to hours for many compositions and is especially useful for comparative screening of dopants, particle size distributions, and binder additives that affect densification and grain boundary behavior.
Caveat: Binder-assisted pressing can introduce artifacts (residual organics) that must be removed or accounted for with thermal-ramp cleaning steps or chemical analysis. IEST recommends validation runs and post-processing controls for binder-based protocols.
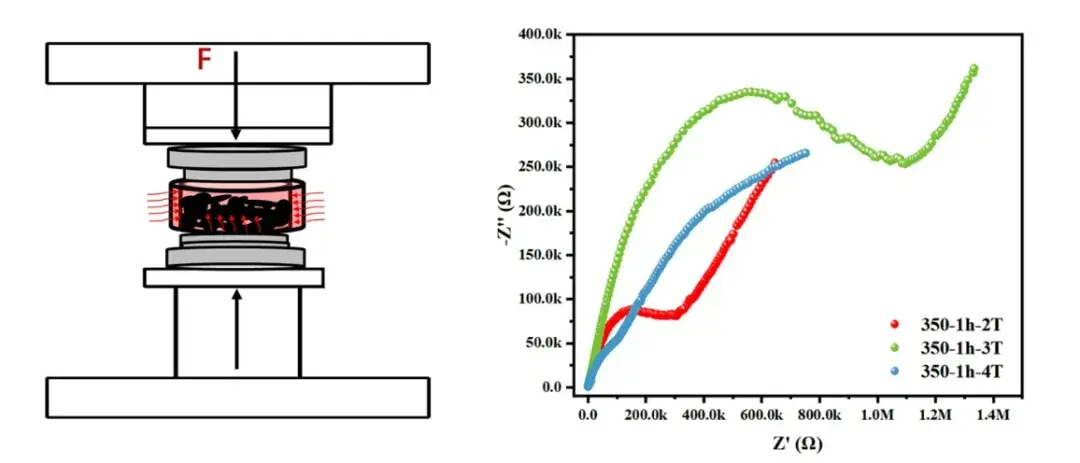
Figure 4. Oxide solid electrolyte hot pressing system and test
6. Semi-solid electrolyte & semi-solid battery testing considerations
Semi-solid batteries blend oxide particles and polymer frameworks with small liquid fractions. To evaluate these systems:
-
Test ionic conductivity and percolation thresholds with powder-compacted and coated architectures at representative pressures.
-
Measure interfacial stability and mechanical integrity over thermal cycling to replicate pack conditions.
-
Use combined testing (SEMS1100 + prototype cell evaluation) to link material ionic conductivity with practical metrics such as charge/discharge polarization and cycle-life behavior.
IEST’s test matrix couples powder and coated film evaluations with prototype cell cycling to derive metrics that better predict semi-solid battery performance.
7. Best practices & recommendations for standardized testing
-
Report full test conditions: pressure, contact method, temperature, pellet thickness and densification rate. These variables strongly affect ionic conductivity.
-
Separate bulk vs interfacial contributions: use high-frequency EIS and equivalent circuit fitting to decouple grain boundary effects.
-
Calibrate rapid methods: validate hot-press results against fully sintered references to build transfer models.
-
Control contamination: ensure binder removal or controlled thermal profiles to prevent organics from skewing ionic conductivity.
-
Measure electronic leakage: confirm ionic dominance so conductivity values are not confounded by electronic conduction.
8. Conclusion
As solid-state batteries become a national strategic priority, advancing the performance and reliability of oxide solid electrolytes will play a key role in enabling next-generation energy storage. IEST remains committed to supporting researchers and industry partners through innovative testing systems such as the SEMS1100 and customized hot-pressing solutions. We welcome collaboration to further refine and standardize testing methodologies for solid-state electrolytes.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.