-
iestinstrument
A New Solution Makes The Battery Recycling Rate Greater Than 99.5%
1. Research Background
In recent years, with the rapid expansion of the new energy vehicle market powered by lithium-ion batteries (LIBs), the number of retired LIBs is expected to increase significantly over the next 5–10 years. Therefore, lithium battery recycling is critical to the sustainability of the industry. Current recycling methods include high-temperature metallurgy, hydrometallurgy, and direct recycling. Direct recycling focuses on restoring the crystalline structure of the materials with low energy consumption and minimal environmental impact.
However, direct recycling methods face significant challenges, particularly in separating electrode materials from the current collectors. The strong adhesion between the active materials and the collectors complicates this process, reducing overall recycling efficiency. Traditional dismantling techniques such as crushing, sorting, and froth flotation often damage the valuable metal foils and contaminate the active materials, making subsequent separation and purification more difficult. These processes require additional high-energy steps and result in environmental pollution. Therefore, ensuring efficient, rapid, and cost-effective separation of current collectors from the active electrode materials is one of the key issues in the development of the lithium-ion battery recycling industry.
2. Work Summary
Recently, the research group of Chao Wang at Tongji University, in collaboration with Yunhui Huang and Ju Li, demonstrated a novel method to efficiently separate electrode materials from current collectors using hydrogen or oxygen microbubbles generated during water electrolysis. By integrating this approach with dry electrode manufacturing processes, the Water Electrolysis Separation (WES) method achieves lower energy consumption, environmental friendliness, shorter processing times, and higher product purity. Specifically, the separation process completes within one minute, with material recycling rates exceeding 99.5% and metal impurity levels below 40 ppm. Nearly 100% of the metal current collectors are recycling while preserving the structural and morphological integrity of the recycled materials. This breakthrough offers an efficient and sustainable solution for battery recycling, balancing environmental protection with high-quality material production. The study was published in the prestigious journal Nature Sustainability, with Ph.D. candidate Fangzhou Yang as the first author.
3. Content Description
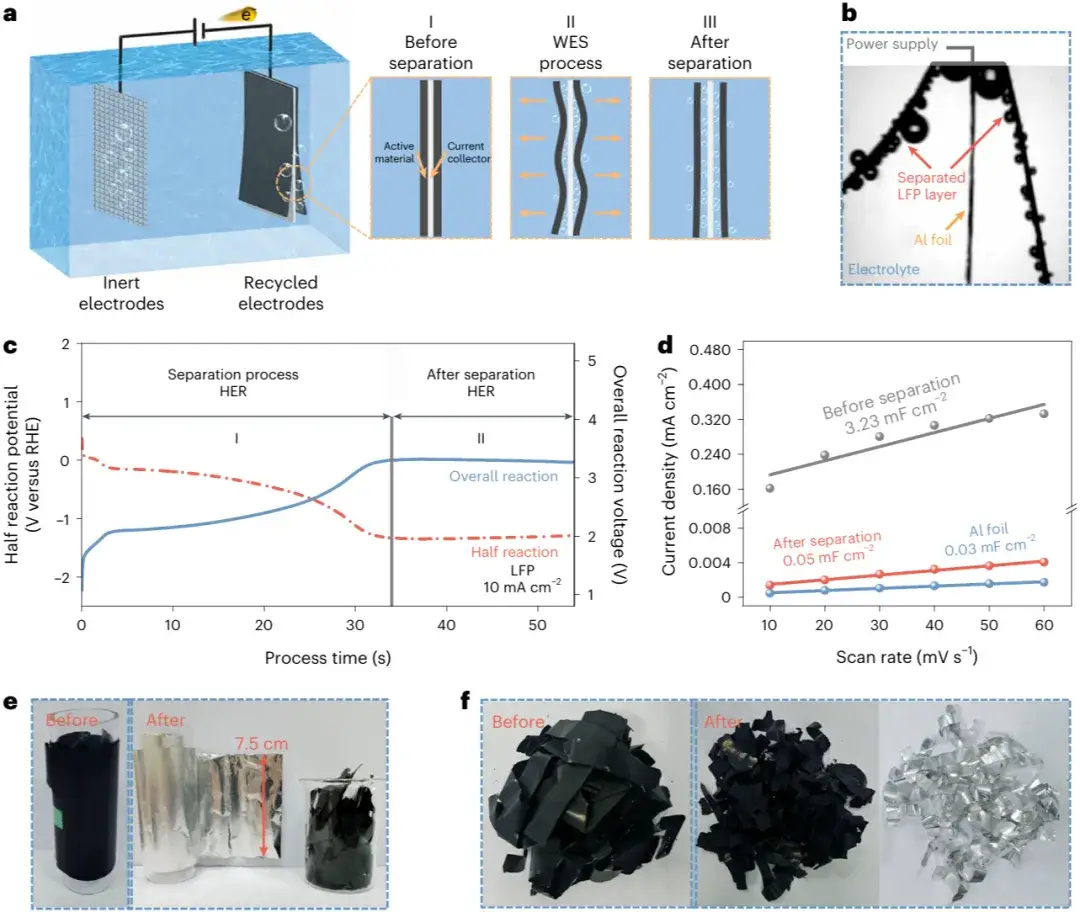
Figure 1. Separation of lithium iron phosphate (LFP) electrodes via the WES method.
In the WES process, hydrogen or oxygen bubbles generated during water electrolysis rapidly delaminate LFP coatings from aluminum foil current collectors. The LFP recycling rate reaches 99.5%, while the current collector recycling rate approaches 100%.
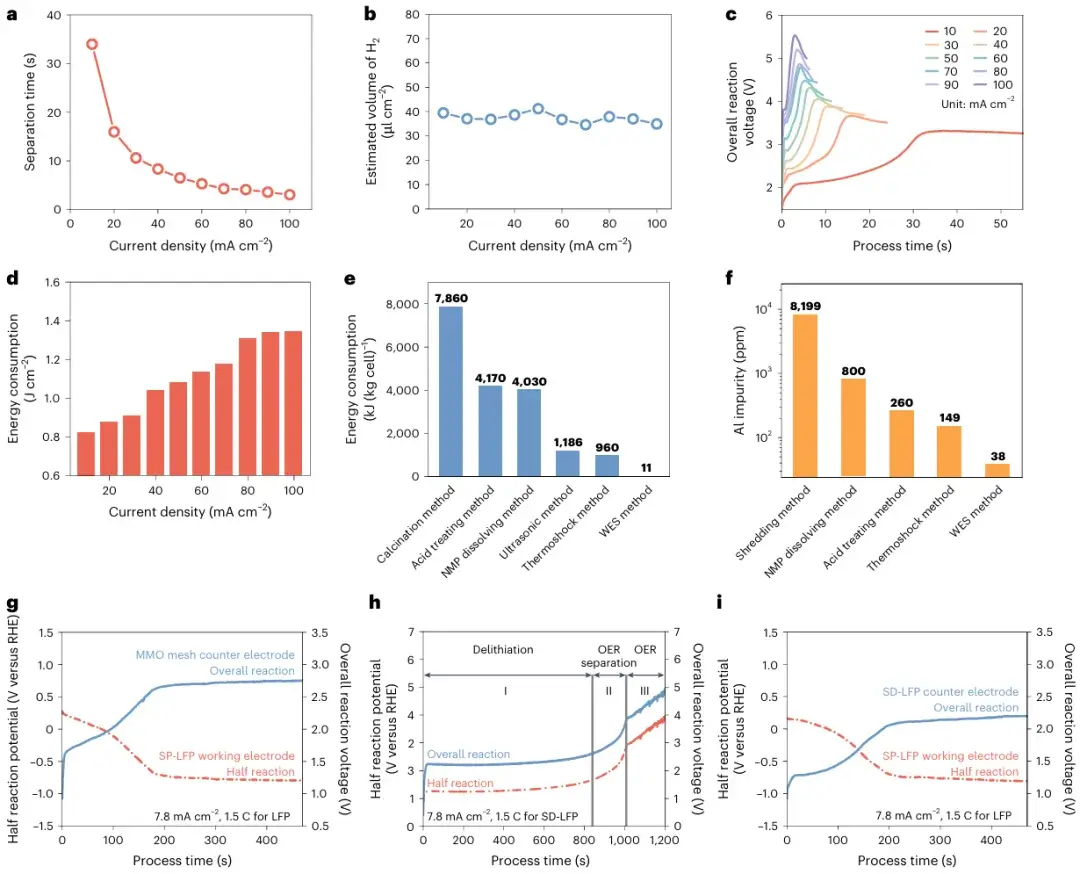
Figure 2. Electrochemical analysis of the WES process for different LiFePO₄ electrode configurations.
Detailed electrochemical tests of the WES method show that, compared to other separation methods, WES achieves lower energy consumption and produces fewer metal impurities in the recycling products. Moreover, different combinations in the WES process can fulfill diverse objectives. For the cathode, the hydrogen evolution reaction enables rapid separation of the electrode material or lithiates the active material. For the anode, either an inert electrode or a LiFePO₄ electrode can be employed for lithium extraction. This versatility demonstrates the broad application potential of the WES process in battery recycling and resource reutilization.
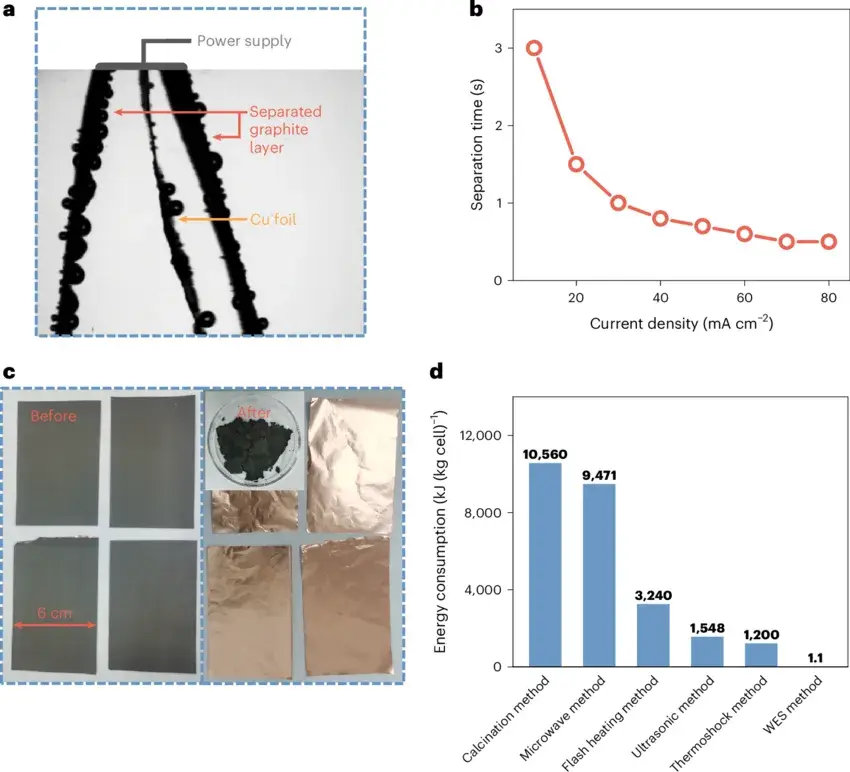
Figure 3. Separation of graphite electrodes using the WES method.
When separating graphite electrodes with the WES process, the delamination force between graphite and its current collector is much lower than that between LiFePO₄ and its current collector. Therefore, the separation time is greatly reduced, and the recycling rate of graphite also reaches 99.5%, with that of the copper foil being approximately 100%. This further demonstrates the high efficiency of the WES process.
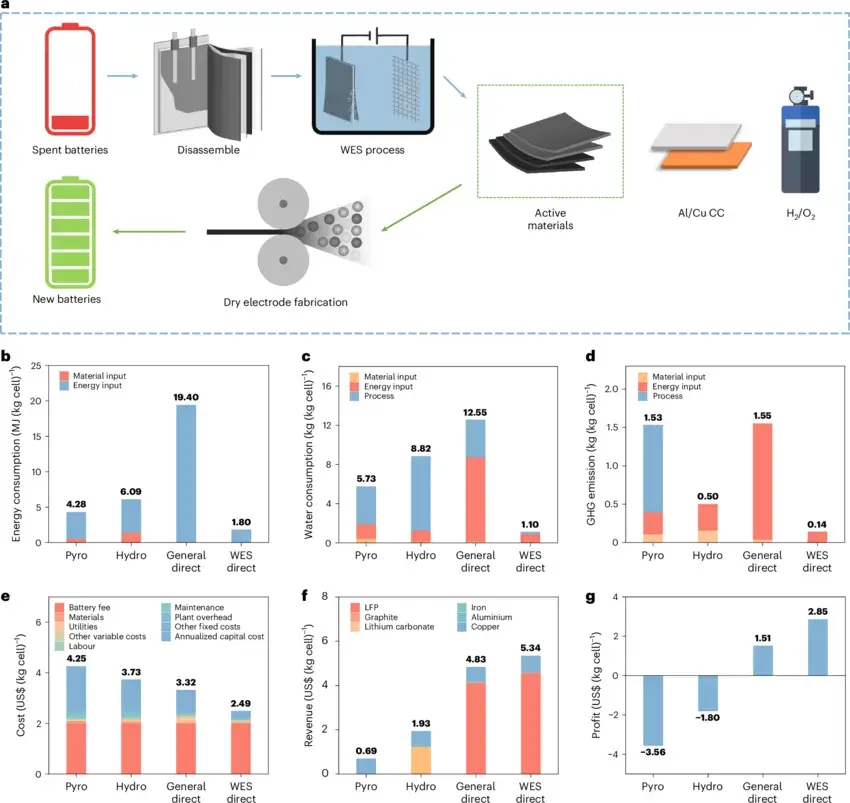
Figure 4. Characterization of dry electrodes prepared from active materials recycling by the WES method.
SEM and XRD analyses reveal that the recycling active material exhibits no significant damage in morphology and retains a complete crystalline structure. Furthermore, the electrochemical performance of dry electrodes prepared from the recycling active material is similar to that of new commercial electrodes. Full cells assembled using the recycling LiFePO₄ edge materials still maintain over 75% capacity after 1000 cycles, indicating that the electrochemical performance is not affected by the recycling process. The various characterizations confirm that the WES method is a non-destructive recycling technique.
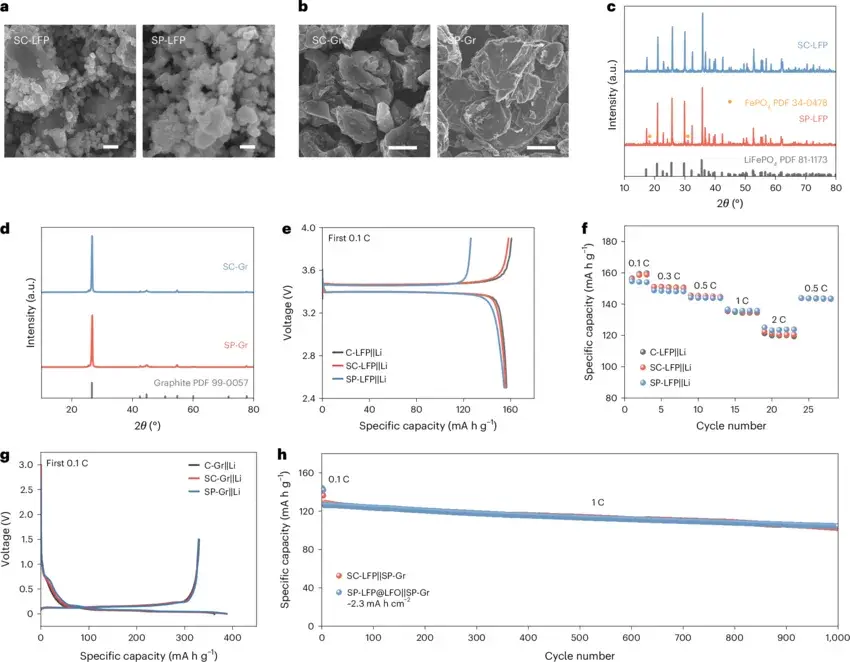
Figure 5. Environmental and economic analysis of different battery recycling methods.
Using the Everbatt model to evaluate environmental friendliness and economic feasibility, the analysis shows that compared with other common recycling methods, the WES process not only consumes the least energy and produces the least waste but also yields the highest profit. Thus, the WES process offers significant advantages in terms of energy efficiency, environmental benefits, and economic performance.
4. Key Conclusions
This study introduces a direct battery recycling method for lithium-ion batteries that combines water electrolysis separation technology with dry electrode remanufacturing. The WES process employs gases generated at the electrode interface during water electrolysis (either from the oxygen evolution or hydrogen evolution reaction) to achieve rapid and effective separation, consuming only water and electricity. In addition, the WES method is compatible with electrodes from various types of retired batteries or production waste, demonstrating exceptional energy efficiency. The separation process is completed within one minute, with a material recycling rate of over 99.5% and metal impurity levels below 40 ppm. Nearly 100% of the metallic current collectors are recycled, preserving the overall structure and morphology of the recycling materials. The multifunctionality of the WES process is evident in that it can achieve multiple targets through different combinations: for the cathode, rapid separation or relithiation of the active materials is achieved via the hydrogen evolution reaction, while for the anode, lithium extraction can be performed using either an inert or LiFePO₄ electrode.
After separation, the recycling active material can be directly used to manufacture dry electrodes. The efficient mixing of the active material with the conductive additive reduces the energy required for the mixing process, making it highly suitable for dry electrode manufacturing. The electrochemical performance of these dry electrodes is comparable to that of freshly manufactured commercial electrodes. Overall, the integration of the WES process with dry electrode technology results in lower energy consumption, reduced environmental impact, shortened processing time, and improved recycling quality. These advantages make our method a practical and environmentally friendly solution for battery recycling and remanufacturing.
5. Original Article
Yang, F. et al. Electrode separation via water electrolysis for sustainable battery recycling. Nature Sustainability (2025).
6. Electrochemical Instruments Provider Recommend:IEST Instrument
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.




