-
iestinstrument
Mechanically and Chemically Co-Robust Ni-Rich Cathodes Enable Ultrahigh Capacity and Prolonged Cycle Life
First Authors: Bo Wang, Kuo Li, Ge Xu, Zihan Zhang
Corresponding Authors: Prof. Jianyu Huang, Prof. Yongfu Tang, Prof. Hui Yang
Used Equipment: IEST Single Particle Force Properties Test System(SPFT2000)
Affiliations:
-
College of Environmental and Chemical Engineering, Yanshan University
-
College of Materials Science and Engineering, Yanshan University
-
School of Aeronautics and Astronautics, Huazhong University of Science and Technology
1. Overview
Ni-Rich layered oxides (NRLOs) are promising high-energy-density cathodes for lithium-ion batteries but suffer from capacity fade and interfacial instability due to chemical/mechanical degradation during cycling. While doping strategies mitigate these issues, their underlying mechanisms remain unclear. This study introduces high-valent niobium (Nb⁵⁺) doping to achieve mechano-chemical co-stabilization in LiNi₀.₈Co₀.₁Mn₀.₁O₂ (NCM811).
Key advances include:
- Grain refinement (∼ceramic/alloy strengthening effect) reducing internal strain and suppressing oxygen loss.
- Stabilized monoclinic phase during H2-H3 transitions and formation of spinel twin boundaries post-cycling.
- Enhanced Li⁺ diffusion (D<sub>Li+</sub> = 3×10<sup>−8</sup> cm²/s vs. 1×10<sup>−8</sup> cm²/s in pristine) via lowered migration barriers.
- Strong Nb–O bonding inhibiting transition metal migration, oxygen release, and rock-salt phase formation.
The 0.5 wt.% Nb-doped NCM811 delivers 233.8 mAh/g at 0.1C and 80.5% capacity retention after 500 cycles (1C), outperforming undoped NCM811 (199.2 mAh/g; 68% retention).
2. Introduction of Grain‑Refinement and Mechanical Performance
We employed low‑content Nb doping (0.5 wt.% Nb) to avoid surface‑coating artifacts. Rietveld refinement of XRD patterns confirms that both pristine NCM811 and NCM811‑0.5Nb adopt the α‑NaFeO₂‑type hexagonal (R‑3m) structure, with Nb doping slightly expanding lattice parameters and unit‑cell volume. The Li⁺/Ni²⁺ mixing ratio decreases from 4.1 % in NCM811 to 1.7 % in NCM811‑0.5Nb. FIB‑SEM reveals that Nb doping markedly refines particle size, increases packing density, and eliminates internal voids, manifesting a “fine‑grain strengthening” effect. Aberration‑corrected STEM imaging shows fused grain boundaries and formation of low‑angle boundaries, which profoundly enhance mechanical robustness. IEST Single Particle Force Properties Test System(SPFT) demonstrate that for 5.1 µm particles, the fracture stress of NCM811‑0.5Nb exceeds that of undoped NCM811, highlighting Nb’s critical role in mechanical reinforcement.
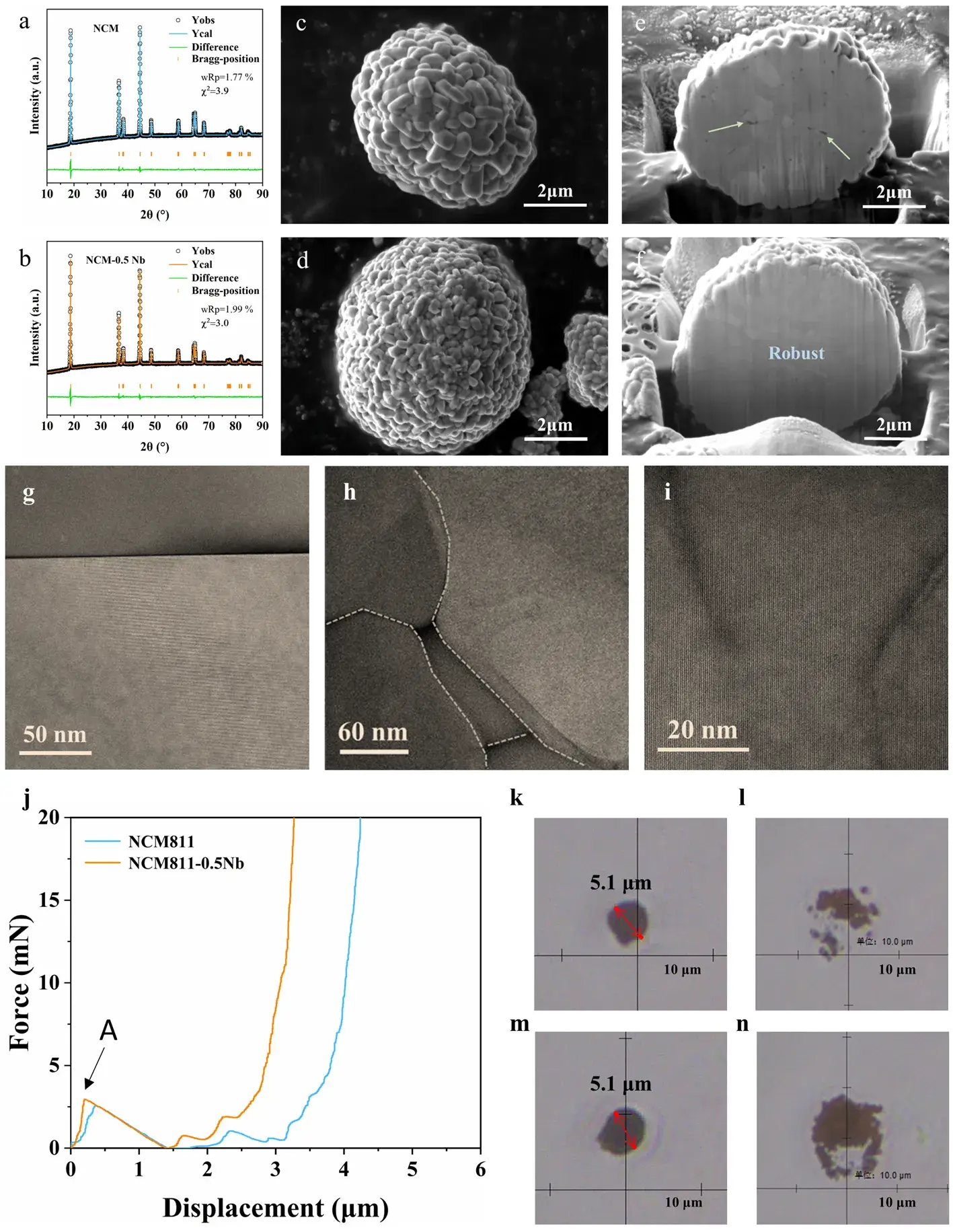
Figure 1. Morphology, microstructure, and mechanical behavior of NCM811 vs. NCM811‑0.5Nb.
3. Electrochemical Performance and Li⁺ Diffusion Enhancement
In the 2.8–4.3 V window, NCM811‑0.5Nb delivers an ultrahigh discharge capacity of 233.8 mAh g⁻¹ at 0.1 C—far surpassing 199.2 mAh g⁻¹ for pristine NCM811. dQ/dV spectra show three redox couples corresponding to H1→M→H2→H3 transitions. The enhanced capacity of NCM811‑0.5Nb predominantly arises from the H1–M–H2 regime. Nb doping lowers peak intensity for H1→M and shifts H2→H3 to lower potentials, indicating accelerated phase‑transition kinetics and improved monoclinic‑phase stability. After 500 cycles at 1 C, capacity retention is 80.5 % for NCM811‑0.5Nb versus 68.0 % for NCM811. GITT measurements reveal that Li⁺ diffusion coefficient (D_Li⁺) increases from 1.0×10⁻⁸ cm² s⁻¹ in NCM811 to 3.0×10⁻⁸ cm² s⁻¹ in NCM811‑0.5Nb. DFT calculations further show that Nb expands interlayer spacing, reducing electrostatic barriers along both octahedral–octahedral direct hops (ODH) and tetrahedral‑site‑mediated hops (TSH). PDOS analysis indicates stronger Ni 3d–O 2p hybridization, with broadened t₂g bandwidth and increased states near the Fermi level, facilitating charge transfer during Ni oxidation.
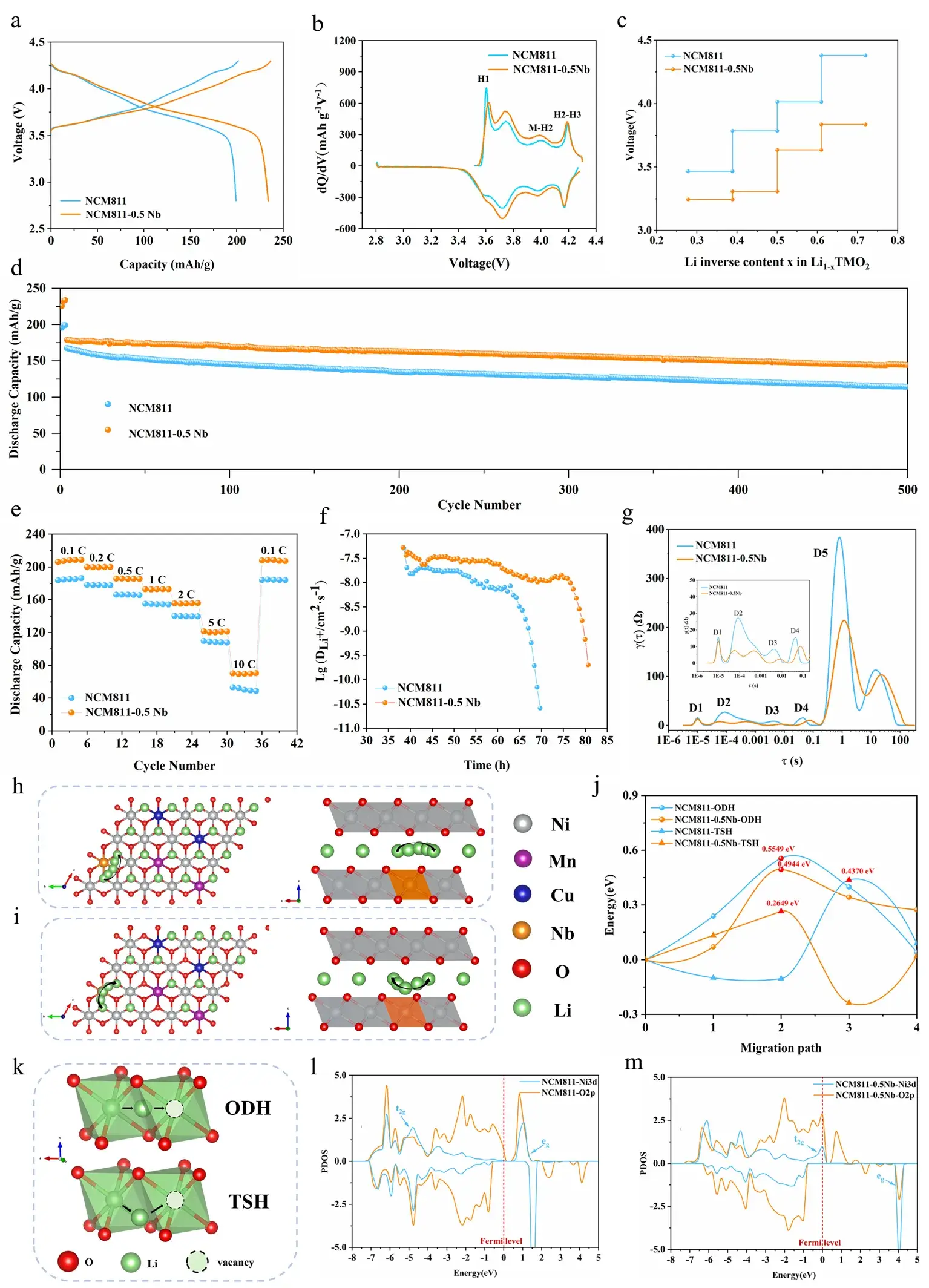
Figure 2. Rate capability and cycle stability of NCM811 vs. NCM811‑0.5Nb, plus DFT‑derived Li⁺ migration pathways and PDOS.
4. Suppression of Fatigue‑Induced Phase Changes
In‑situ XRD reveals that repeated H2–H3 cycling induces lattice fatigue in pristine NCM811, whereas Nb doping significantly mitigates this degradation, maintaining phase coherence throughout cycling.
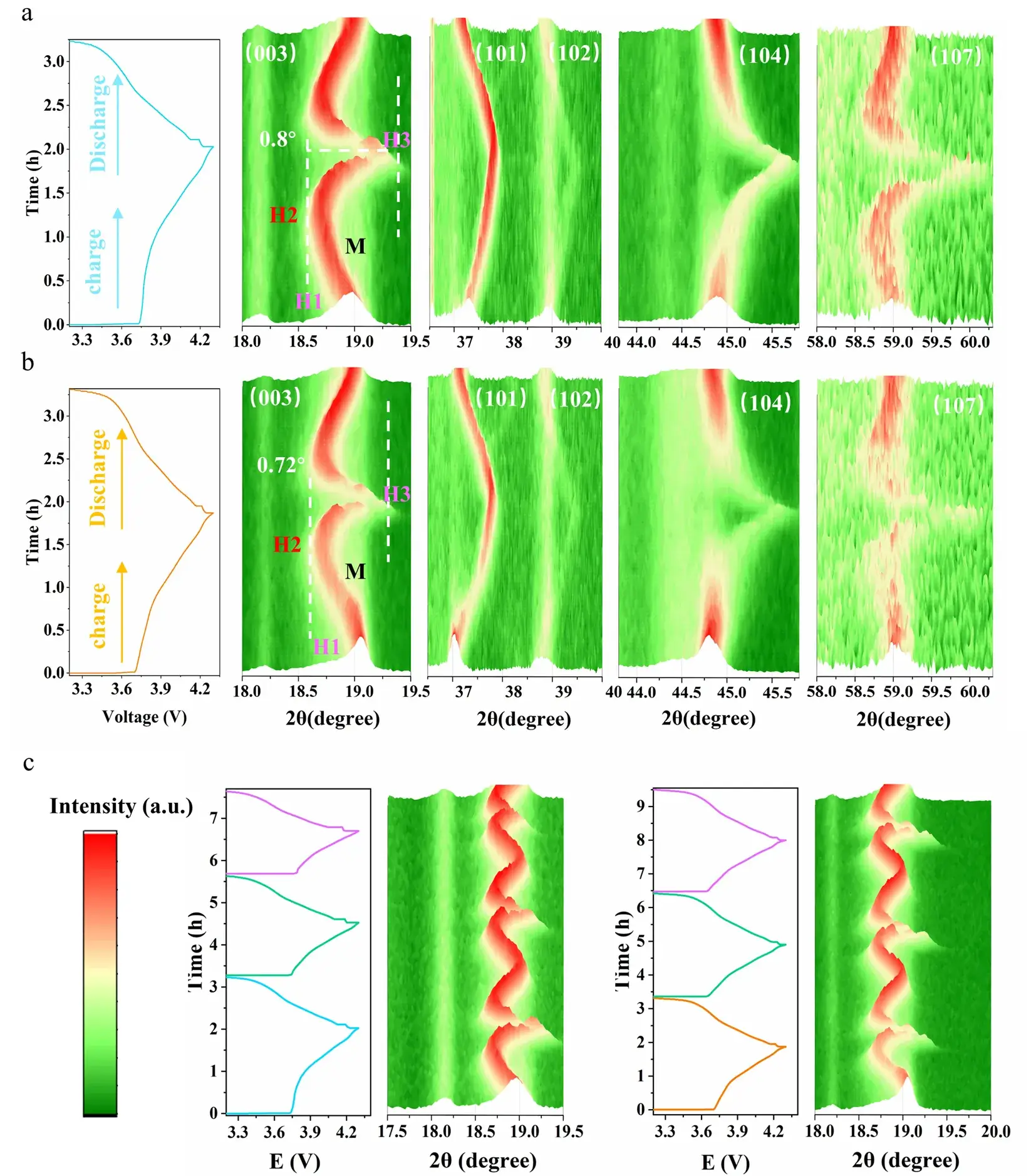
Figure 3. In‑situ XRD monitoring of phase evolution under galvanostatic cycling.
5. Microcrack Formation and Stabilization Mechanisms in Ni-Rich Cathodes
The study identifies microcrack propagation as a primary degradation mode in Ni-rich cathodes during extended cycling. Cross-sectional SEM imaging after 500 cycles reveals significantly more cracks in NCM811 compared to Nb-doped NCM811-0.5Nb.
Microstructural Evolution Analysis (HAADF-STEM)
-
NCM811: Extensive intergranular cracks initiate at secondary particle cores and propagate along grain boundaries (GBs). These cracks facilitate electrolyte penetration, exacerbating interfacial side reactions. GBs act as strain concentration sites, accumulating cyclic stress and causing severe particle fracturing.
-
NCM811-0.5Nb: No observable cracks after 500 cycles; secondary particles maintain structural cohesion.
Stabilization Mechanisms Enabled by Nb⁵⁺ Doping
-
Grain Refinement Strengthening:
-
Nb doping reduces primary particle size, inducing a “grain refinement” effect analogous to ceramic/alloy strengthening.
-
Increased GB density dissipates internal strain energy, suppresses crack propagation, and enhances mechanical robustness.
-
-
Lattice Oxygen Stability (EELS Analysis):
-
O K-edge spectra exhibit two characteristic peaks:
-
Pre-edge (P): Arises from electronic transitions from O 1s to unoccupied states hybridized with TM 3d orbitals.
-
Main peak (M): Corresponds to transitions from O 1s to Ni 4sp bands.
-
-
NCM811: Reduced P/M ratio at GB surfaces indicates oxygen vacancies due to surface-preferential oxygen release. This accelerates disordered phase formation and degrades Li⁺ diffusion kinetics.
-
NCM811-0.5Nb: Uniform TM oxidation states and higher P/M ratio confirm stabilized lattice oxygen. Strong Nb–O bonding mitigates oxygen release, disrupting the degradation cascade.
-
-
Spinel Twin Boundary Formation:
-
Post-cycling observations reveal epitaxial growth of spinel twin boundaries between layered-structure grains.
-
These boundaries—comprising spinel phases epitaxially bonded to adjacent layered domains—originate from Nb-induced grain refinement and regulated interfacial stress.
-
They constrain complete disordering and alleviate abrupt internal strain during H2↔H3 phase transitions, significantly enhancing long-term cyclability.
-
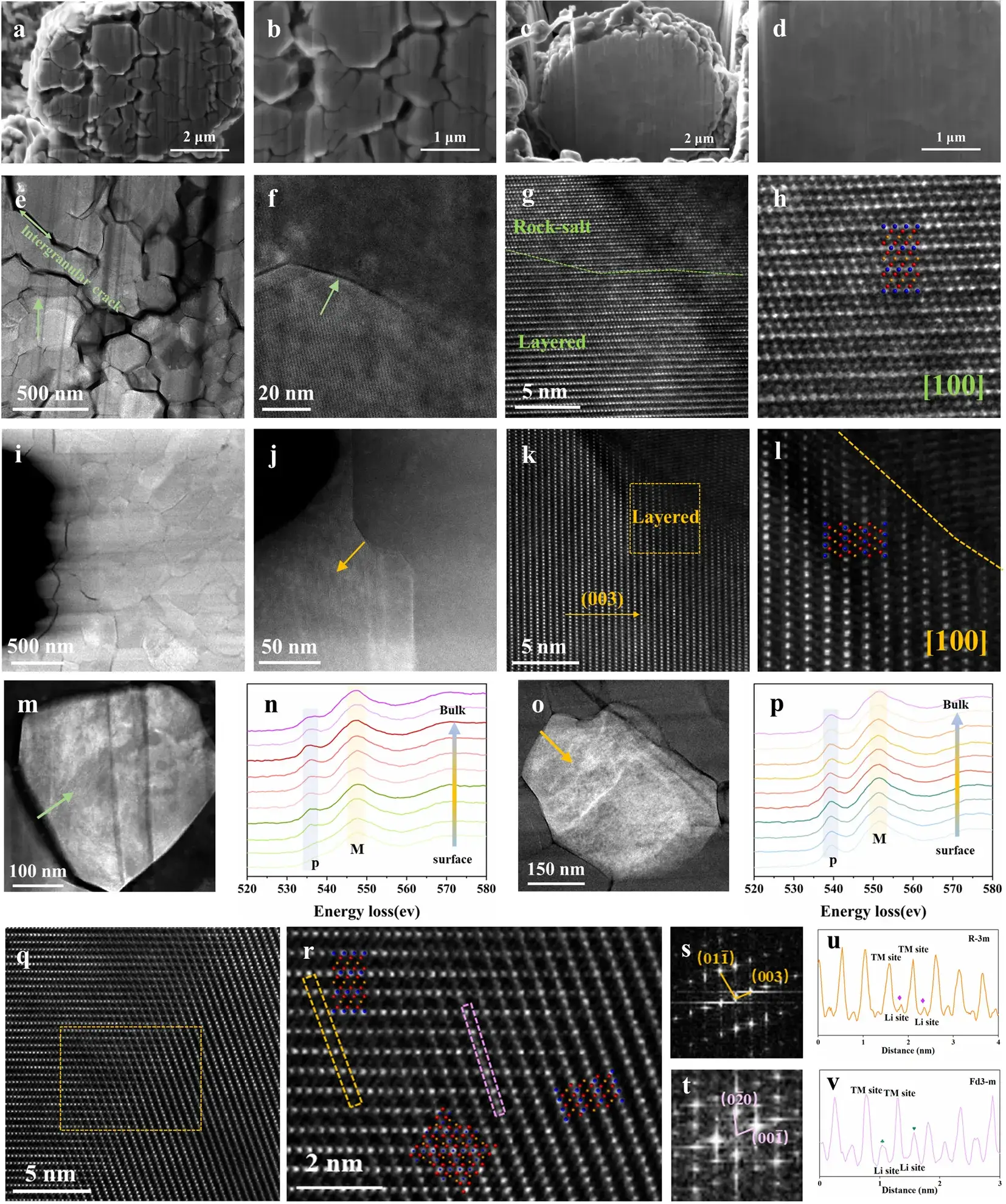
Figure 4. Morphological and structural evolution after cycling.
To further investigate stress and damage evolution in NCM811 and NCM811-0.5Nb secondary particles during delithiation, the authors performed chemo-mechanical simulations in ABAQUS. Figure 5 illustrates Li⁺ extraction propagating from the core to the surface of secondary particles, facilitated by interlayer channels and grain boundaries (GBs) within primary grains.
Simulation Methodology:
-
Boundary Condition: A normalized Li concentration CLi = 0.5.CLi = 0.5 was applied at the particle surface to ensure numerical convergence.
-
Concentration Range: CLiCLi varied from 1.0 (fully lithiated) to 0.5 (partially delithiated), with CLi=0CLi=0 representing complete delithiation.
Key Findings:
-
NCM811:
-
Random grain orientations generated highly heterogeneous Li⁺ concentration distributions during delithiation.
-
This inhomogeneity induced severe mismatch stress at GBs, triggering intergranular damage and crack initiation.
-
Stress relaxation occurred as cracks propagated along GBs.
-
-
NCM811-0.5Nb:
-
Nb doping reduced grain orientation disparities, yielding a more homogeneous Li⁺ concentration profile.
-
Improved concentration uniformity alleviated GB mismatch stress and mitigated particle degradation.
-
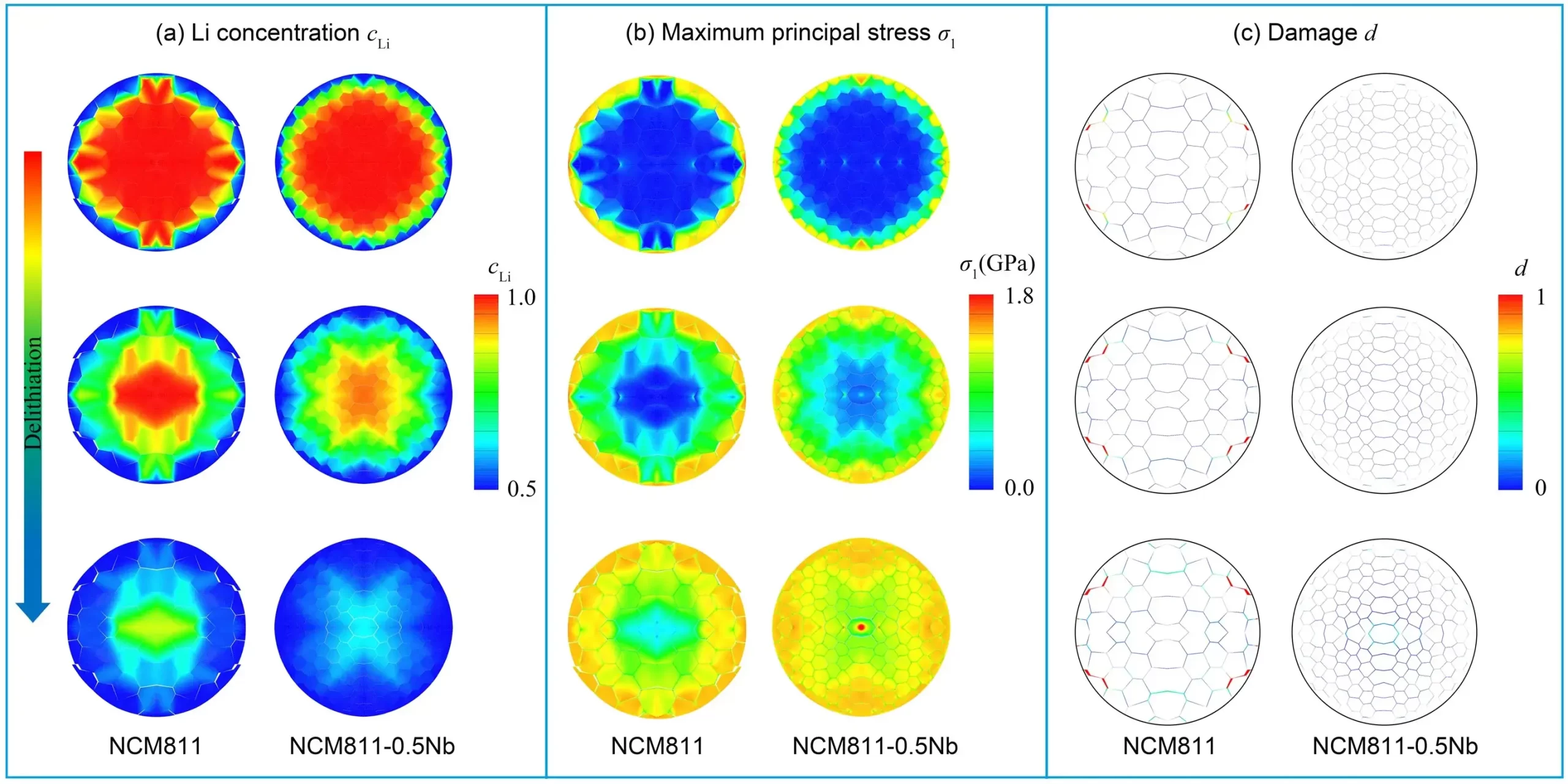
Figure 5. Finite‑element profiles of normalized Li concentration and resulting stress fields during delithiation.
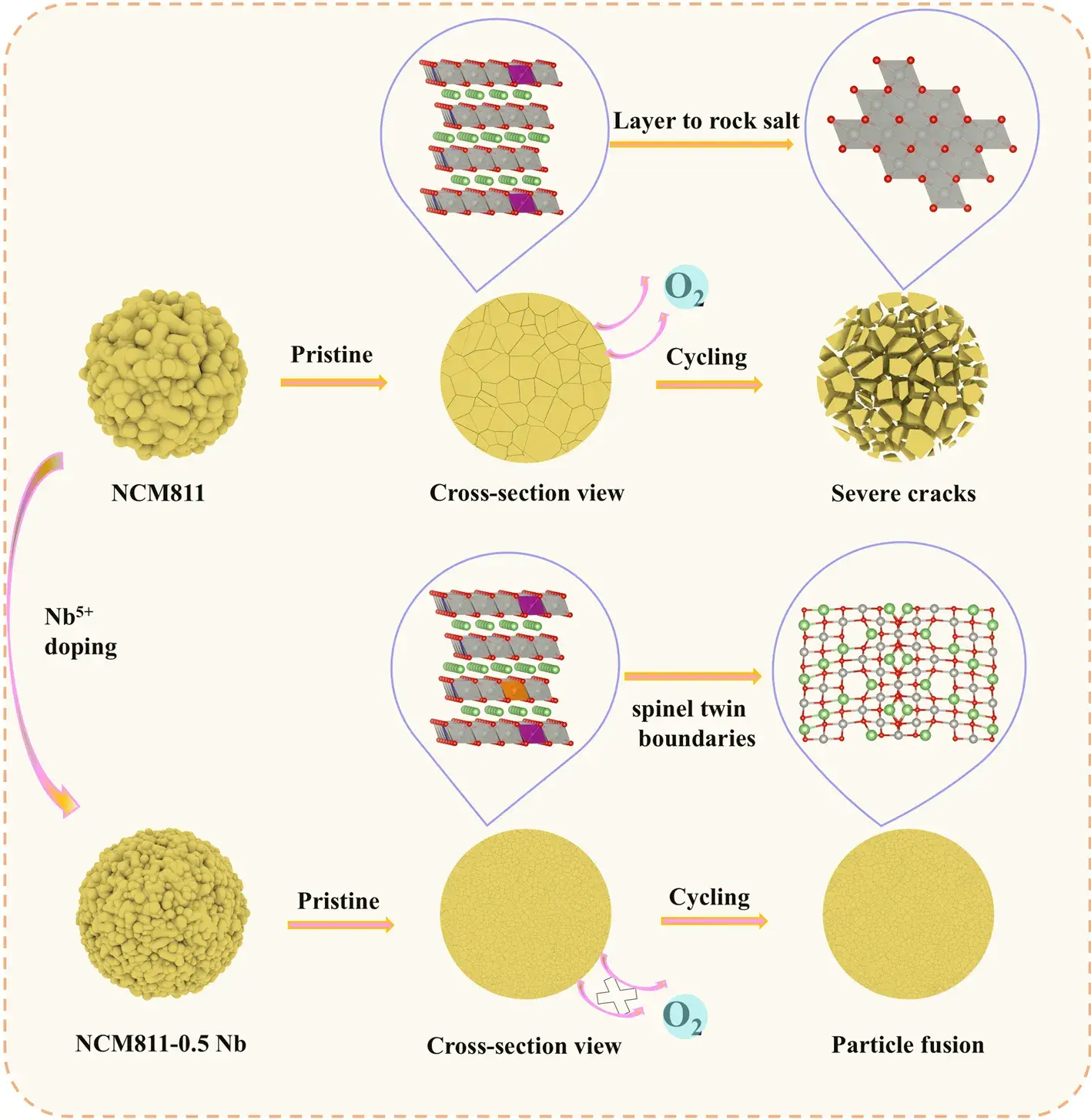
Figure 6. Schematic of failure mechanism in NCM811 and Nb‑doped improvement strategy.
6. Summary and Outlook
In summary, the coupled mechanical and chemical effects of Nb⁵⁺ doping on Ni‑rich layered oxide cathode(NRLOs) have been elucidated. Mechanically, Nb doping refines primary crystallites and fuses grain boundaries, effectively relieving internal stresses during the H2–H3 phase transition and preventing microcrack initiation throughout cycling. This observation is corroborated by coupled chemo‑mechanical simulations in ABAQUS. Chemically, Nb incorporation strengthens Nb–O bonds and expands the interlayer spacing, suppressing transition‑metal (TM) migration from the TM layer into the Li layer, thereby reducing Li/Ni antisite defect formation during synthesis and electrochemical cycling. First‑principles calculations confirm that Nb doping lowers the Li⁺ migration energy barrier within the layered lattice, enhancing Li⁺ diffusivity. Post‑cycle microstructural analysis reveals the formation of spinel‑type twin boundaries, which further facilitate Li⁺ transport while inhibiting additional crack propagation and particle pulverization.
The simple Nb‑doping strategy achieves high capacity, rapid charging capability, prolonged cycle life, and maintained safety, without compromising the performance requirements of next‑generation electric vehicle batteries. Nonetheless, long‑term cycling stability of high‑Ni layered cathodes remains a challenge. Future efforts will focus on designing and implementing strategies to control grain size, grain orientation, and lattice chemistry to further increase specific capacity and, from a chemo‑mechanical perspective, enhance structural robustness of layered oxide cathodes. Although scaling these approaches for large‑scale production poses significant hurdles, this work provides critical scientific guidance for the development of high‑performance layered oxide cathodes for high‑energy‑density storage applications.
7. Testing Instruments Recommendation
IEST Single Particle Force Properties Test System(SPFT2000)
Application:
- Testing the crushing strength of battery material particles
- Can be used to evaluate the pressure resistance of the material
- Guide the rolling process
- Materials with high mechanical strength will have better subsequent cycle stability.
8. References
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.