-
iestinstrument
Binder, Conductive Additive, Electrode Active Material, Current Collector: Who Controls the Critical Factors of Electrode Flexibility?
1. Abstract
An engineering review of how electrode active material, conductive additives, binders and current collectors determine electrode flexibility
2. Introduction
Lithium-ion batteries are essential for modern energy storage, powering everything from electric vehicles to consumer electronics. The electrode, a core component, directly dictates a battery’s energy density, cycle life, and safety. A key mechanical property is electrode flexibility—the ability of an electrode sheet to deform under stress without cracking. Poor flexibility leads to electrode fracture and active material pulverization during cycling, degrading electrical contact, increasing resistance, and accelerating capacity fade. It also raises the risk of internal short circuits under mechanical shock. Therefore, understanding and controlling the factors that influence electrode flexibility is critical for developing durable, safe batteries.
An electrode is a composite comprising active material, conductive agents, binders, and a current collector (Figure 1). These components work synergistically to determine its mechanical behavior. This article analyzes the distinct role of each in controlling electrode flexibility.
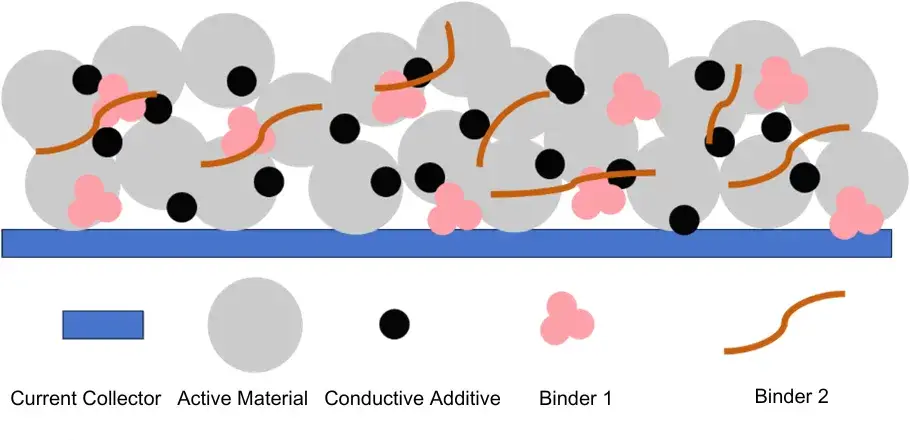
Figure 1. Composition of electrode components
3. Electrode Active Material: The Dominant Driver of Volume Change and Stress Distribution
The electrode active material is the principal source of lithiation-driven volumetric change, and therefore it largely controls stress generation inside the electrode stack.
-
Materila Type: Different active materials exhibit varying degrees of volume change during charge/discharge. For instance, silicon-carbon anodes can experience volume expansion rates as high as 300%, while graphite anode materials typically expand around 10%. Volume changes induce stress; localized stress can cause internal fracture of anode particles or delamination of the Solid Electrolyte Interphase (SEI) layer (Figure 2). Both particle fracture and SEI layer delamination expose fresh electrode surfaces, leading to continuous SEI formation and accelerated electrode aging.
-
Particle Morphology: Spherical or near-spherical active material particles are beneficial for enhancing electrode flexibility. This is attributed to their higher packing density and more uniform stress distribution, which effectively buffer the stress generated by volume changes. Conversely, plate-like or needle-like particles tend to create stress concentration points, leading to localized cracking within the electrode.
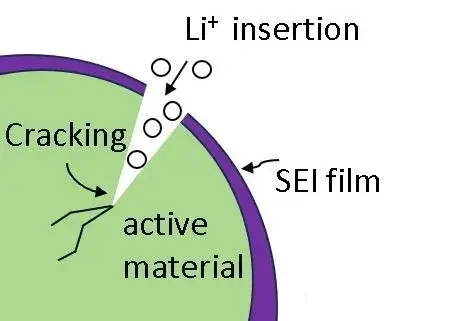
Figure 2. Schematic of material cracking due to stress from volume change.
4. Conductive Additive: The “Double-Edged Sword” of Conductive Network and Mechanical Strength
Conductive additives form the electronic percolation network that enables current collection, yet they also influence mechanical strength and flexibility.
-
Type: One-dimensional (e.g., carbon nanotubes) or two-dimensional (e.g., graphene) conductive agents can create a robust, interconnected network. This network bridges active material particles and the current collector, often enhancing both conductivity and mechanical reinforcement.
-
Content & Dispersion: An optimal amount of well-dispersed conductive agent forms a continuous network that improves strength and flexibility. However, excessive amounts can displace active material, reducing energy density, and may introduce brittleness. Agglomerated conductive particles create weak spots and local stress concentrators, detrimental to flexibility.
5. Binder: The “Adhesive” for Electrode Components
The binder is a vital component of the electrode. Its main function is to bond the electrode active material, conductive additive, and current collector, imparting mechanical strength to the electrode. The type, content, and molecular weight of the binder influence electrode flexibility.
-
Type: Different binders exhibit distinct mechanical properties and adhesion strengths. For example, polyvinylidene fluoride (PVDF) binders offer high mechanical strength but relatively poor flexibility, making them prone to cracking during charge-discharge cycles. In contrast, styrene-butadiene rubber (SBR) binders possess a high elastic modulus, which effectively absorbs stress induced by volume changes and enhances electrode flexibility.
-
Content: Insufficient binder content compromises the mechanical strength of the electrode, increasing its susceptibility to cracking. Conversely, excessive binder content reduces the proportion of active material in the electrode, negatively impacting the energy density of the battery.
-
Molecular Weight: High molecular weight binders can form a more robust bonding network, improving the mechanical strength of the electrode. However, this may come at the cost of reduced flexibility.
6. Current Collector: The Structural Backbone
The current collector serves as the carrier for electron transport within the electrode. Its material, thickness, and surface treatment affect electrode flexibility.
-
Material: Metals like copper foil and aluminum foil possess inherent ductility and flexibility, providing fundamental mechanical support to the electrode and contributing to its overall flexibility. However, if the current collector itself is excessively thick, has low purity, or contains numerous impurities, its flexibility may be compromised, adversely affecting the electrode’s flexibility.
-
Thickness: Increasing current collector thickness enhances the overall strength of the electrode but may reduce flexibility. Thicker current collectors require greater force to bend and are more susceptible to crack formation.
-
Surface Treatment: Roughening the surface of the current collector increases the contact area with the active material, enhancing adhesion and creating a more robust bond. This generally improves electrode flexibility. However, excessive roughness can lead to non-uniform distribution of the active material, potentially decreasing flexibility.
7. Innovative Method for Quantifying Electrode Flexibility
Electrode flexibility is a key factor influencing the cycle life and safety performance of lithium-ion batteries. By optimizing electrode composition and manufacturing processes, highly flexible electrodes can be developed, thereby improving battery cycle life and safety, and advancing lithium battery technology.
To accurately assess electrode flexibility, IEST Instrument has developed a testing instrument based on stress-strain curve analysis (Figure 3). This method secures the electrode, applies displacement, and measures the force and displacement (force-displacement curve) in real-time during deformation, enabling quantitative analysis of flexibility. This technology provides crucial data support for optimizing electrode composition and processing, facilitating the development of high-flexibility electrodes.
Figure 3. IEST Battery Electrode Flexibility Testing System (BEF1000) Equipment
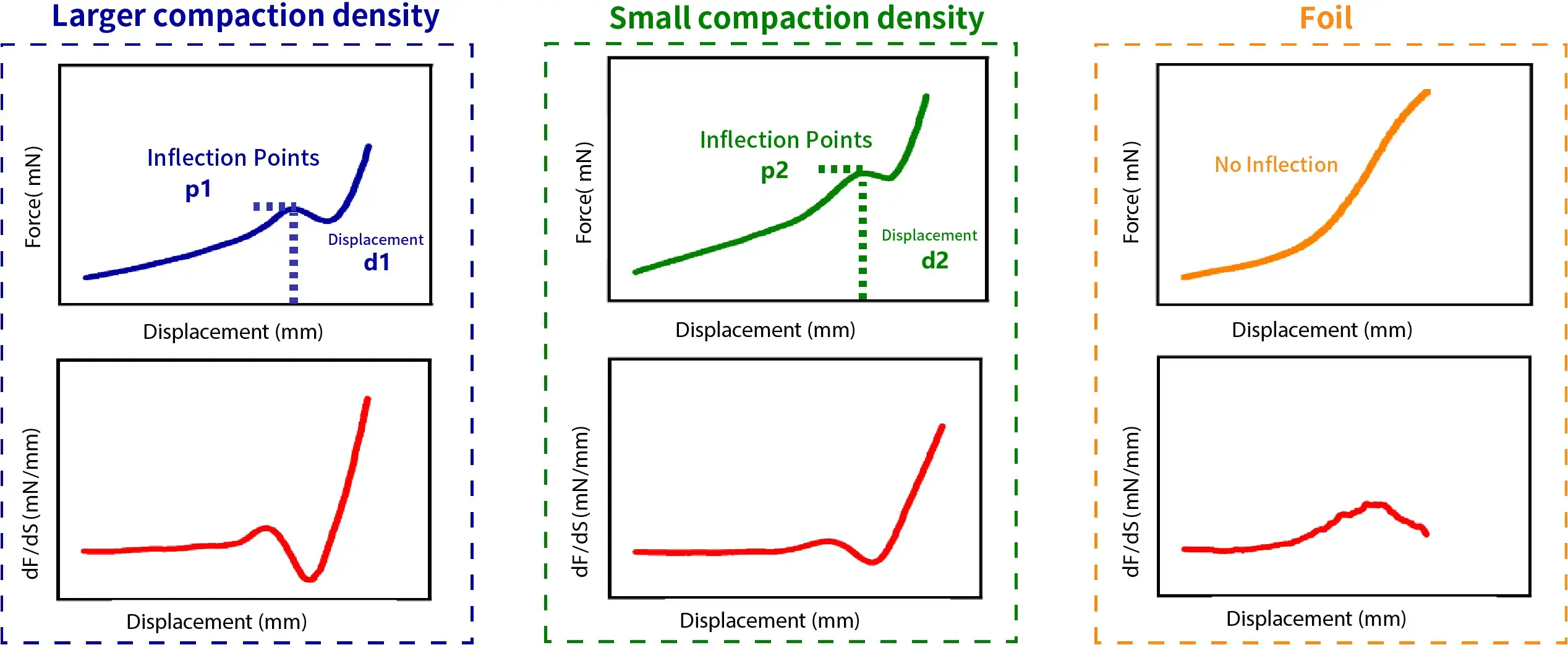
- Compare the shape of the curves: Higher flexibility electrodes may exhibit smoother curves with no distinct abrupt changes or inflection points.
- Compare the slopes: Electrodes with better flexibility typically show a smaller initial slope in their curve, indicating they undergo larger deformation under the same applied force.
- Analyze the fracture point: Generally, electrodes exhibiting a larger compression displacement at the fracture point possess superior flexibility.
- Observe the first derivative: Electrodes with poorer flexibility may exhibit larger peaks or sharp changes in their first derivative curve.
8. Practical Recommendations — How to Design for Electrode Flexibility
-
Start with the active material. Where possible, select particle morphologies and secondary-particle architectures that reduce excessive local strain; for Si-rich anodes, prefer engineered yolk-shell or carbon-scaffolded particles.
-
Design the percolation network. Use 1D/2D conductive additives judiciously to create resilient, stress-dissipating networks; ensure high dispersion quality to avoid rigid clusters.
-
Choose binders for resilience. For high-expansion chemistries favor elastic binders (SBR, elastomeric copolymers) or hybrid formulations that combine adhesion and reversible strain. Adjust binder content to meet mechanical gates without unduly sacrificing active material fraction.
-
Tune current collectors and surface treatment. Select foil thickness and a controlled surface roughness that maximize adhesion while preserving bendability.
-
Define process windows by mechanical failure points. Use measured yield/fracture values to set limits for calendering pressure, slitting tension and winding lines. Do not rely on machine settings alone—use material-level test data to define safe operating envelopes.
8. Summary
Electrode flexibility is a core indicator for battery performance and safety. By synergistically optimizing the selection and ratios of active material, conductive additive, binder, and current collector, and leveraging advanced testing methodologies, the comprehensive performance of electrodes can be significantly enhanced. This lays a solid foundation for the development of next-generation lithium-ion batteries with high energy density and long cycle life.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.