-
iestinstrument
Analysis of Electrical Conductivity and Compression Properties of Hard Carbon and Graphite Materials
1. Background
With the rapid advancement of the new energy sector, demand for efficient and cost-effective energy storage continues to grow. While lithium-ion batteries dominate the market, issues related to raw material scarcity and cost have spurred interest in sodium-ion batteries as a promising alternative.
A critical challenge in sodium-ion technology is the selection of suitable anode materials. Hard carbon vs graphite is a common trade-off: although graphite is widely used in lithium-ion systems, it exhibits poor performance in sodium-ion batteries due to the thermodynamic instability of sodium intercalation into graphite layers. In contrast, hard carbon has emerged as a leading candidate, offering a high specific capacity (around 300 mAh/g) and low working potential (~0.1 V). Beyond electrochemistry, powder physical properties — electrical conductivity, compaction density and thickness rebound — strongly influence electrode manufacture and final cell performance.
This article presents a focused graphite analysis and test graphite carbon comparison in terms of conductivity, compaction density, and mechanical rebound—key properties influencing electrode processing and battery performance. Using the PRCD3100 powder resistivity & compaction density tester to help R&D and process engineers choose and optimize materials.
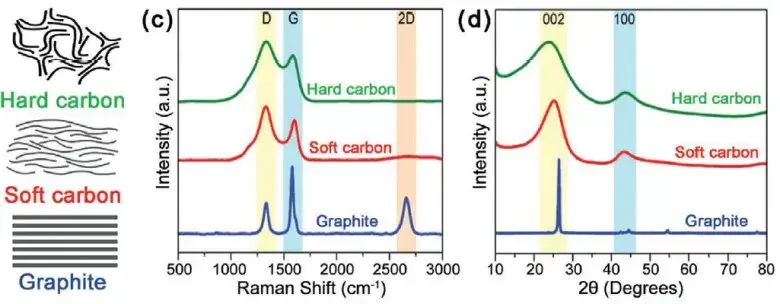
Figure 1. structural differences between graphite, hard and soft carbon2
2. Test Method
2.1 Test Equipment
The IEST Powder Resistivity & Compaction Density Tester (PRCD3100) was used to measure the electrical and mechanical properties of two types of graphite and two types of hard carbon materials. The system, shown in Figure 2, applies controlled pressure while recording resistivity, thickness, and density in real time.
Figure 2. (a)PRCD3100 appearance diagram; (b)PRCD3100 structure diagram
2.2 Test Parameters
Samples were subjected to pressure ranging from 5–200 MPa, with increments of 20 MPa and a holding time of 10 seconds at each step. This protocol allows systematic observation of how each material responds to compression and decompression.
3.Test Results
3.1 Electrical Conductivity and Compaction Density
As illustrated in Figure 3, both graphite samples showed significantly higher electrical conductivity and compaction density compared to hard carbon samples. These differences can be attributed to the highly ordered layered structure of graphite, which facilitates electron mobility, while the disordered and porous nature of hard carbon hinders efficient charge transport.
The compaction density of graphite was also notably higher, approaching its true density of 2.3 g/cm³ under high pressure. Hard carbon, with its inherent microporosity, showed lower densification due to the presence of voids and structural defects that resist full compression even at 200 MPa.
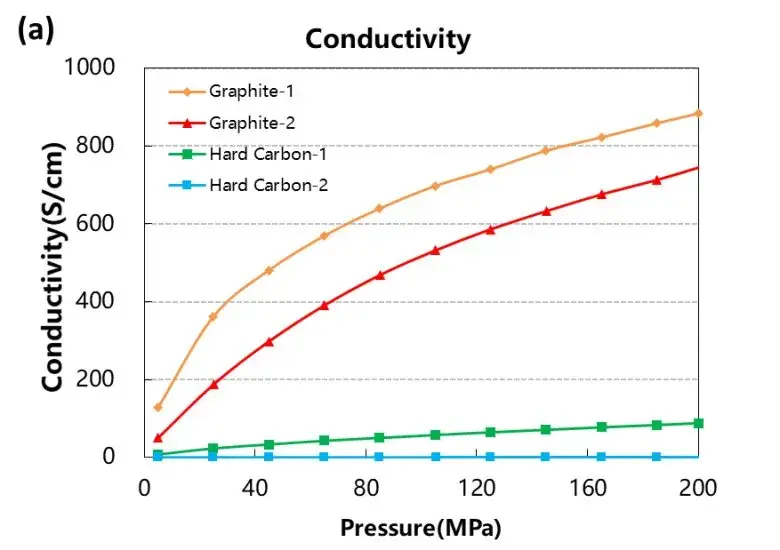
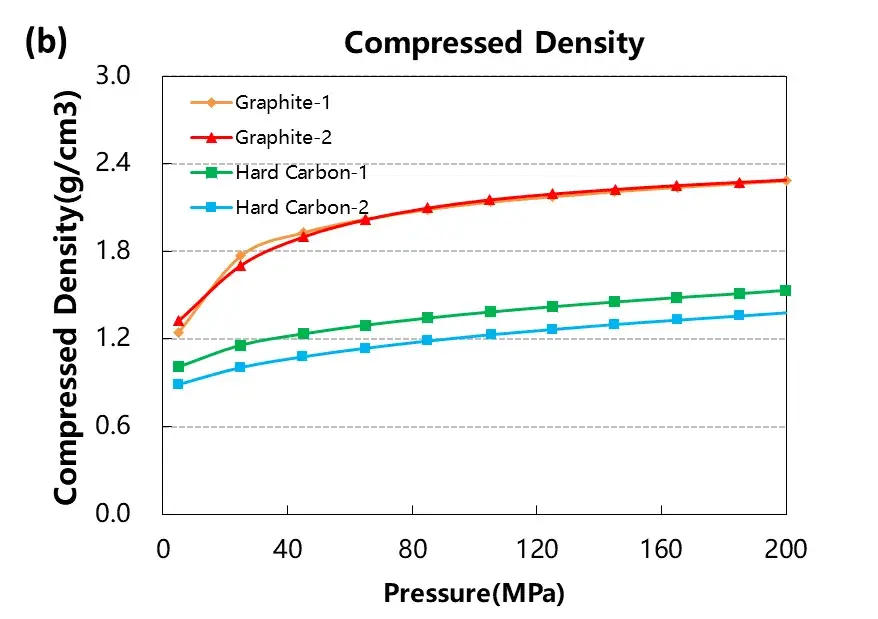 Figure 3. Electrical conductivity and compaction density curves of the four graphite and hard carbon materials.
Figure 3. Electrical conductivity and compaction density curves of the four graphite and hard carbon materials.
3.2 Rebound Behavior and Stress-Strain Response
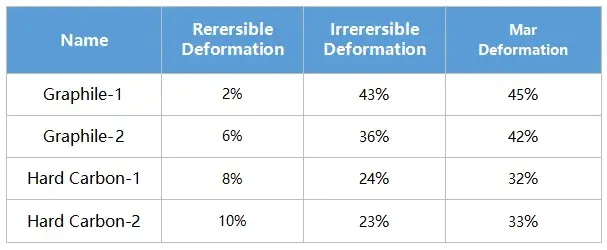
The rebound characteristics after compression are critical in electrode calendering and affect final electrode thickness. Figure 4 and Table 1 summarize the deformation data:
-
Graphite exhibited minimal rebound beyond 50 MPa, indicating stable elastic recovery.
-
Hard carbon showed greater reversible and irreversible deformation, with continued rebound even at higher pressures.
These results suggest that graphite offers better dimensional stability during electrode manufacturing. The stress-strain curves further confirm that graphite has a lower compression modulus, enabling higher packing density with less elastic recovery compared to hard carbon.
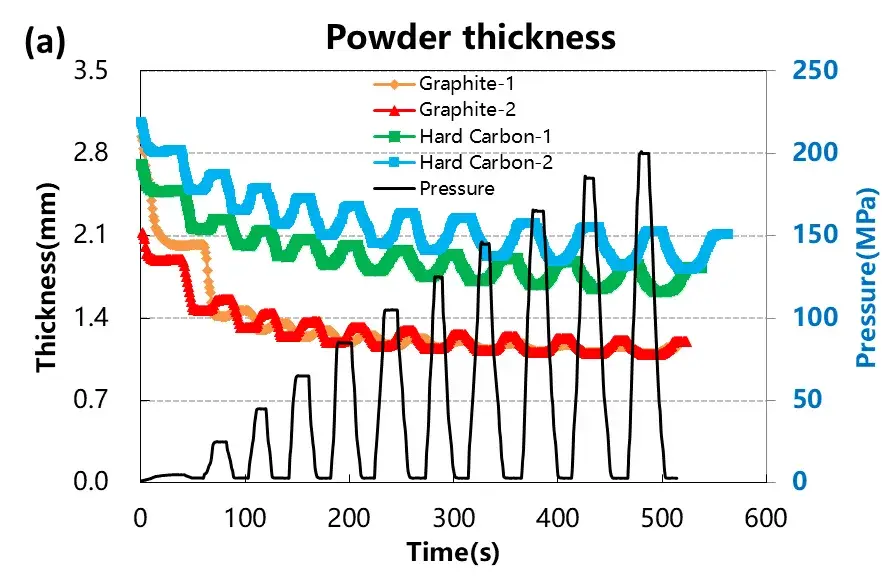
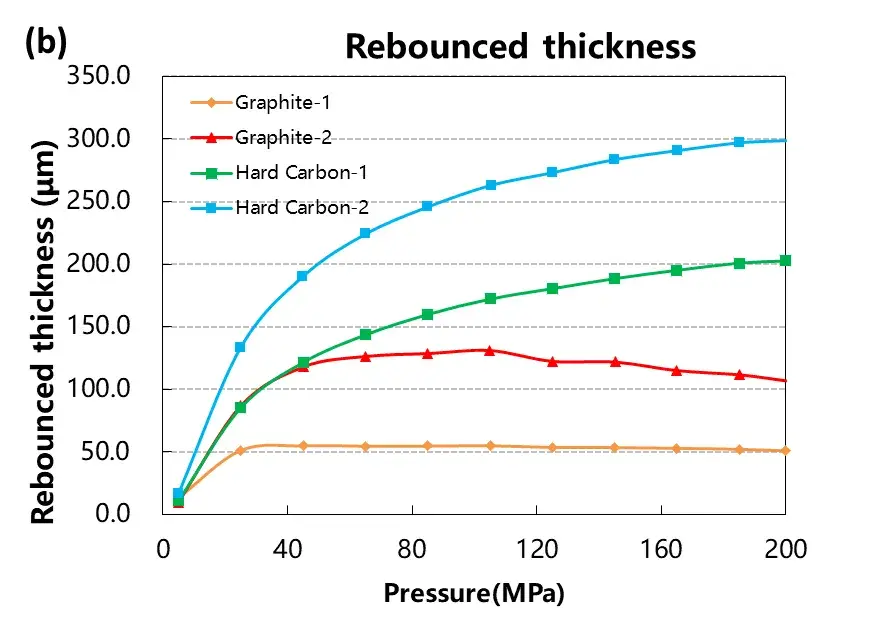
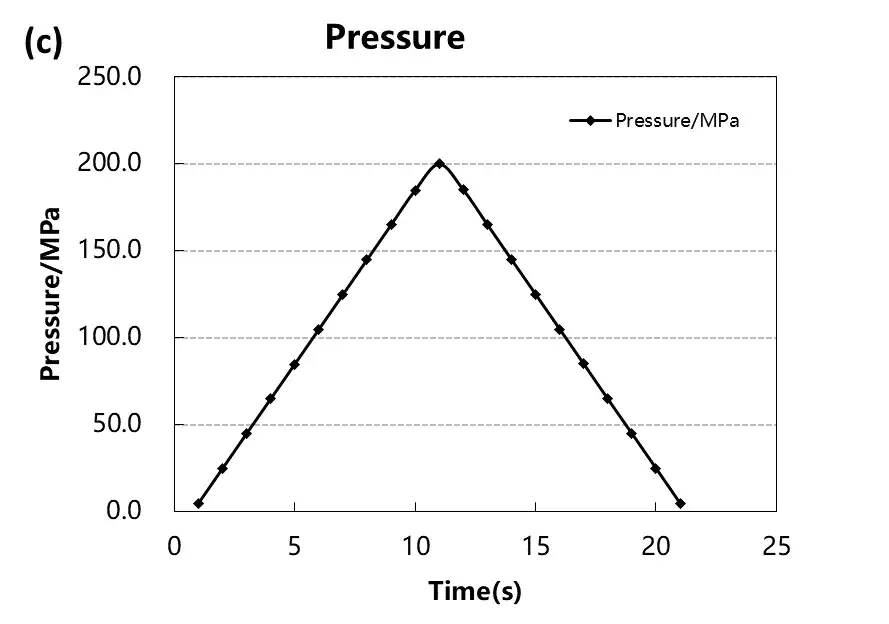
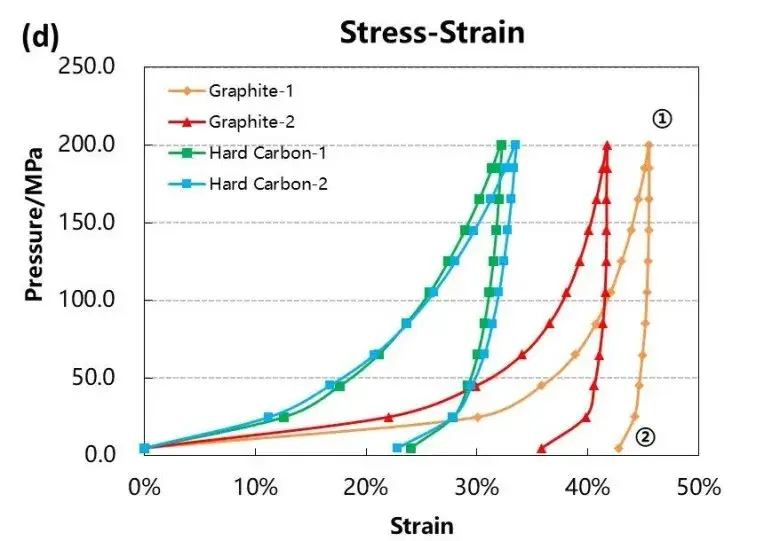
Figure 4. Stress-strain curves during pressurization and depressurization of four materials
Table 1.Summary of morphometric data for the four materials
3.3 Structural Explanations for Performance Differences
As shown in Figures 5 and 6, graphite consists of ordered graphene layers held by van der Waals forces, allowing electrons to move freely parallel to the layers, resulting in high conductivity.
In contrast, hard carbon possesses a highly disordered structure containing micropores, cross-links, and oxygen functional groups that disrupt electron flow and reduce conductivity. Its random and rigid microstructure also leads to higher rebound and lower compaction under pressure.
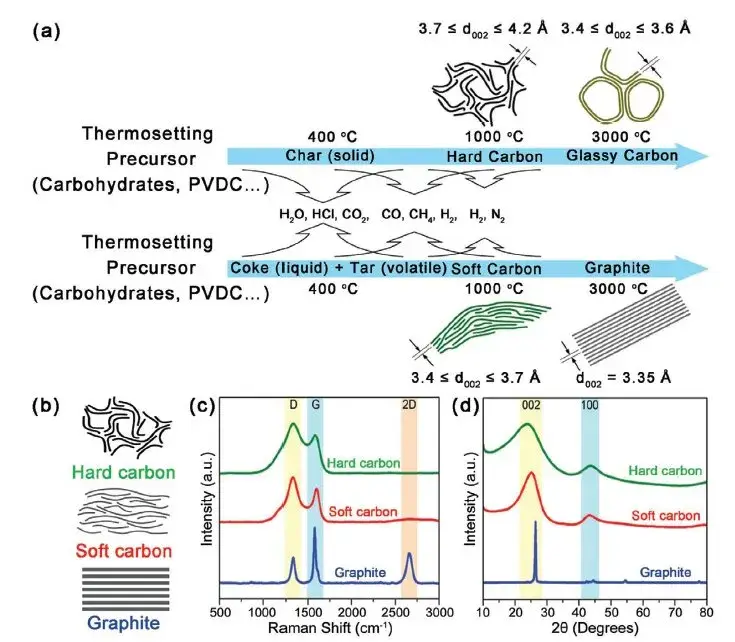
Figure 5. Formation and microstructure of graphite, hard carbon and soft carbon materials2
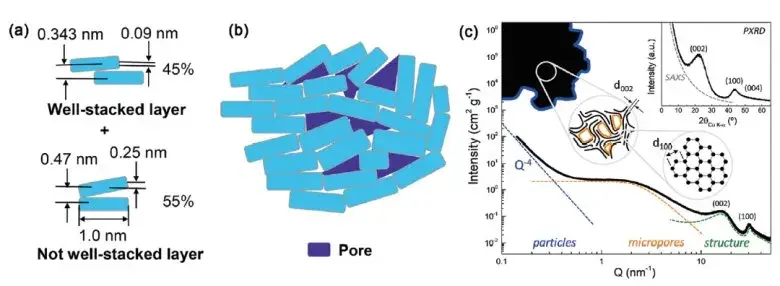
Figure 6. Schematic diagram of the structural analysis of the hard-carbon materials2
5. Summary
This study provides a systematic graphite analysis and comparison with hard carbon using the PRCD3100 testing platform. Key findings include:
-
Graphite outperforms hard carbon in electrical conductivity and compaction density.
-
Hard carbon exhibits greater elastic rebound, which may pose challenges in electrode processing.
-
Microstructural differences—order versus disorder—are the fundamental reasons for the divergence in properties.
When selecting materials for sodium-ion or lithium-ion batteries, it is essential to consider not only storage performance (sodium/lithiation capacity) but also these critical mechanical and electrical properties. This test graphite carbon study underscores the need for holistic material evaluation to guide application-specific choices in battery design and manufacturing.
6. References
[1] Hu Yongsheng,Lu Jiaxiang,Chen Liquan,etc.,Sodium Ion Battery Science and Technology, Science Press,2020,134-137.
[2] Lijing Xie, Cheng Tang, Zhihong Bi,et al. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective.Adv.Energy Mater.2021, 2101650.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.




