-
iestinstrument
Analysis of Formation Temperature Impact on Battery Cell Volume Expansion
1. Introduction
Formation is a critical manufacturing step for lithium-ion cells: its primary purpose is to generate a stable solid electrolyte interphase (SEI film) on the negative electrode to electronically isolate the electrode while allowing ionic transport¹ ². The quality of the SEI formed during this step strongly influences later cycle performance and lifetime. In addition, formation reactions often produce gas and induce electrode volume changes; therefore, formation temperature is a key process parameter that couples electrochemistry, interphase chemistry, and cell swelling. In this article, In-Situ Gassing Volume Monitor Analyzer (GVM) is used to test the in-situ volume measurements performed on NCM523/graphite cells at different formation temperatures and explains how formation temperature influences SEI film properties and the timing and magnitude of cell volume swelling.
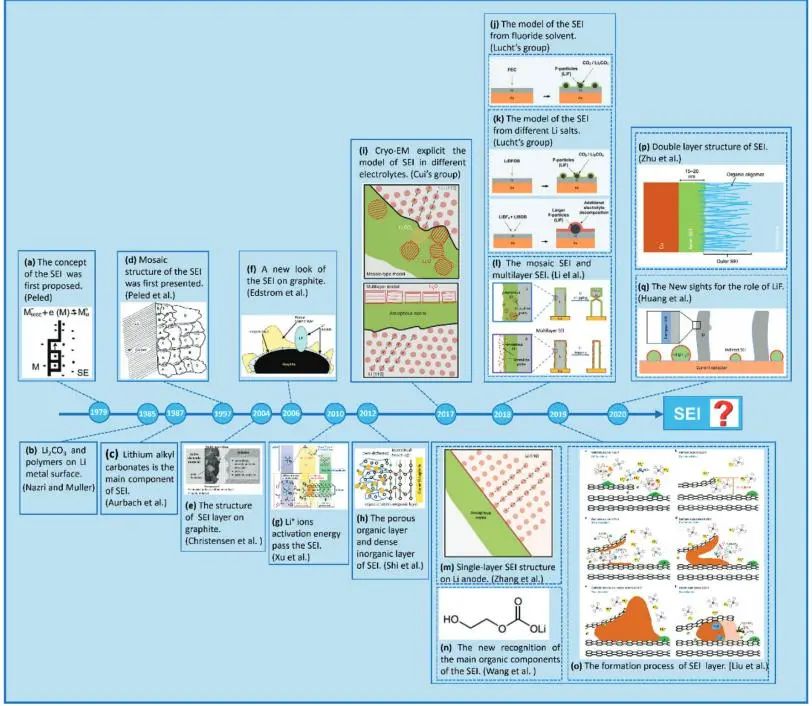
Figure 1. Research progress and timeline of SEI on graphite and lithium metal surfaces¹
2. Experimental Equipment and Test Methods
2.1 Experimental Equipment
Model GVM2200(IEST), the test temperature range is 20°C~85°C, and it supports dual-channel (2 batteries) simultaneous testing. The appearance of the equipment is shown in Figure 2.
Figure 2. Appearance of GVM2200 Equipment
2.2 Test Information
Cells with an NCM523/Graphite system were used, charged at 0.5C CC to 4.2V, with a theoretical capacity of 2400mAh.

Figure 3. Test Cells
2.3 Test Method
Cells were initially weighed (m₀), placed into the instrument channels, and the MISG software was initiated. The software was configured with the corresponding cell ID and sampling frequency parameters, automatically recording data for volume change, temperature, current, voltage, and capacity.
3. Formation Temperature Schedule
Five formation temperatures were compared: 25°C, 45°C, 55°C, 65°C, and 85°C, following the process in Figure 4(a). For each temperature point we ran five parallel cells following the same formation procedure to produce comparable volume-expansion and differential capacity data sets are shown in Figures 4(b) and 4(c). The resulting volume and dQ/dV profiles were used to assess gas evolution, phase-transition behavior and SEI evolution as a function of formation temperature.
4. Observations — Timing and Magnitude of Volume Expansion
As formation temperature increased, the onset of measurable cell expansion occurred earlier in the charge profile. Volume growth accelerated and reached a near-stable maximum when the cell voltage approached approximately 3.7 V, followed by a slight contraction during the constant-voltage stage. Higher temperatures produced larger total gas evolution and earlier expansion, indicating that elevated formation temperature shifts and accelerates the reactions that contribute to swelling.
Notably, when formation temperature exceeded 55°C, the first phase-transition peak in the differential capacity curves became noticeably sharper. This sharpening is consistent with more abrupt electrochemical events and suggests that high-temperature formation drives more intense SEI-related reactions (for example, faster reduction of solvent components and more rapid decomposition pathways).
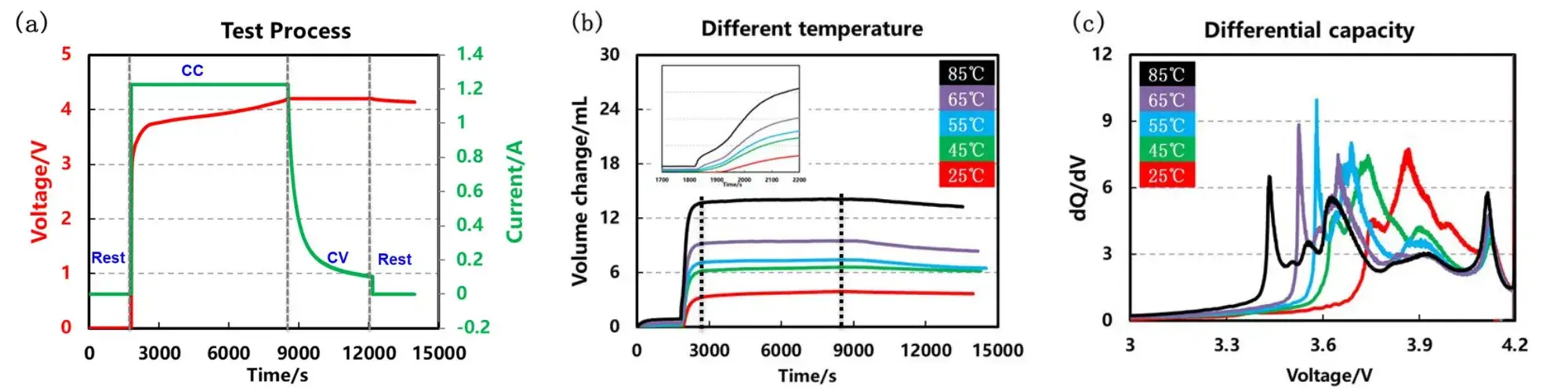
Figure 4. Cell formation process, volume swelling and differential capacity curves
5. SEI Film Formation: Competing Processes and Temperature Dependence
SEI formation is governed by two opposing processes: SEI growth (net formation) and SEI dissolution (loss into the electrolyte). Experimental evidence shows SEI growth is largely tied to electrochemically induced solvent reduction and is relatively less sensitive to temperature. In contrast, higher temperatures accelerate dissolution of the initially formed SEI components into the electrolyte. As a result, SEI films produced at different temperatures possess distinct compositions and morphologies.
At elevated temperatures, organic SEI components dissolve more readily in organic electrolyte, favoring an SEI enriched in inorganic species (for example, LiF, Li₂CO₃, RCO₂Li, and other carbonates) that are less soluble. This compositional shift reduces the mechanical compliance of the SEI and decreases the electrode’s ability to accommodate volume changes, thereby exacerbating measurable cell swelling and interface instability under high-temperature formation.
Conversely, SEI formed at lower temperatures tends to be denser and more compact. While compact SEI may reduce gas-producing side reactions, it can also exhibit lower ionic conductivity and higher polarization; at very low formation temperatures this can limit lithium transport and increase the risk of localized lithium plating. Thus, there is a trade-off: low temperature → dense but less ionically conductive SEI; high temperature → more inorganic, less compliant SEI and more gas evolution.
6. Mechanistic Interpretation: Transport, Viscosity and Interfacial Stress
Formation temperature alters several coupled physical properties:
-
Electrolyte viscosity and ionic conductivity: Higher temperature reduces viscosity and increases ionic conductivity, which increases Li⁺ transport rates and reduces cell polarization during formation.
-
Electrode diffusion kinetics: Elevated temperature increases Li diffusion within active materials, shifting phase boundaries and causing phase-transition peaks to shift to lower voltage (left shift in dQ/dV).
-
Interfacial chemical kinetics: Higher temperatures accelerate side-reactions and SEI dissolution, increasing gas production and modifying SEI composition toward inorganic-rich layers that are mechanically stiffer.
Together, these effects explain why higher formation temperature generally reduces polarization and can improve some formation metrics, yet beyond an optimal range it degrades SEI mechanical integrity and increases swelling and irreversible side reactions.
7. Quantitative Trends and Practical Temperature Window
The in-situ volume data show a clear trend: higher formation temperature leads to earlier and larger volume increases. Excessive formation temperature also correlates with stronger gas evolution and sharper early phase peaks in the dQ/dV curves, particularly above 55°C. Because overly high formation temperatures accelerate volatile component loss and damage SEI structure, the industry commonly adopts 45°C–70°C as a practical formation window that balances ionic transport, SEI quality and gas evolution control.
8. Practical recommendations for formation process control
-
Target a balanced formation temperature: Based on the measured trends, select a formation temperature within 45°C–70°C for NCM523/graphite cells as a starting point; fine tune within this window according to electrolyte formulation and electrode porosity.
-
Monitor in-situ volume or gas evolution during pilot runs: Real-time volume monitoring (e.g., GVM2200) helps detect excessive gas generation or abrupt SEI-related events and informs whether formation temperature adjustments are needed.
-
Consider SEI chemistry when changing temperature: Higher temperature tends to favor inorganic-rich SEI; if an elastic, organic-rich SEI is desired (for high-expansion anodes), moderate formation temperatures and additive strategies may be preferable.
-
Beware of volatile electrolyte loss: Formation temperatures near the high end of the range (e.g., 85°C) can accelerate the loss of low-boiling components and trigger secondary reactions; therefore limit exposure time at high temperature or revise electrolyte formulation accordingly.
9. Summary
Formation temperature strongly controls two intertwined outcomes: the chemical and mechanical character of the SEI film and the timing/magnitude of cell volume expansion during formation. This study utilized a controllable dual-channel in-situ gas volume monitor reveals that higher temperatures accelerate expansion and gas evolution and shift electrochemical phase transitions, while also changing SEI composition toward inorganic-rich, less compliant films — particularly above 55°C. For NCM523/graphite cells tested here, an industry-typical formation window of 45°C–70°C provides a reasonable compromise between reduced polarization and preservation of SEI integrity. These quantitative, in-situ measurements can guide process engineers to optimize formation temperature together with formation rate, pressure and electrolyte/additive selection.
10. References
[1] Jian Tan, John Matz, Pei Dong, Jianfeng Shen, and Mingxin Ye. A Growing Appreciation for the Role of LiF in the Solid Electrolyte Interphase. Adv. Energy Mater. 2021.DOI:10.1002/aenm.202100046
[2] Wei Wenfei, Zhong Kuan, Jiang Shiyong. Effect of the formation temperature on lithium-ion battery performance[J]. Energy Storage Science and Technology, 2018, 7(5): 908-912.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



