-
iestinstrument
Analysis of the Effect of Formation Temperature On The Volume Expansion of Cells
1. Preface
Formation is a key process in the production and manufacturing process of lithium-ion batteries. The purpose of formation is to generate SEI on the surface of the negative electrode to isolate electrons and conduct ions¹ ². The quality of SEI film formation directly affects the subsequent cycle rate performance of the battery. Therefore, controlling the appropriate formation conditions (formation temperature, charge rate, applied pressure, etc.) is a very important production step. The SEI film formation process will be accompanied by an increase in battery volume. On the one hand, it is due to the gas products of the film formation reaction, and on the other hand, it is due to the swelling of the negative electrode structure after lithium-ion are extracted from the cathode electrode and inserted into the anode electrode.
In this paper, In-Situ Gassing Volume Monitor Analyzer (GVM) is used to test the in-situ formation volume of NCM523/graphite cells (theoretical capacity 2400mAh) with different formation temperatures and analyze the influence of temperature on the battery cell swelling of the volume.
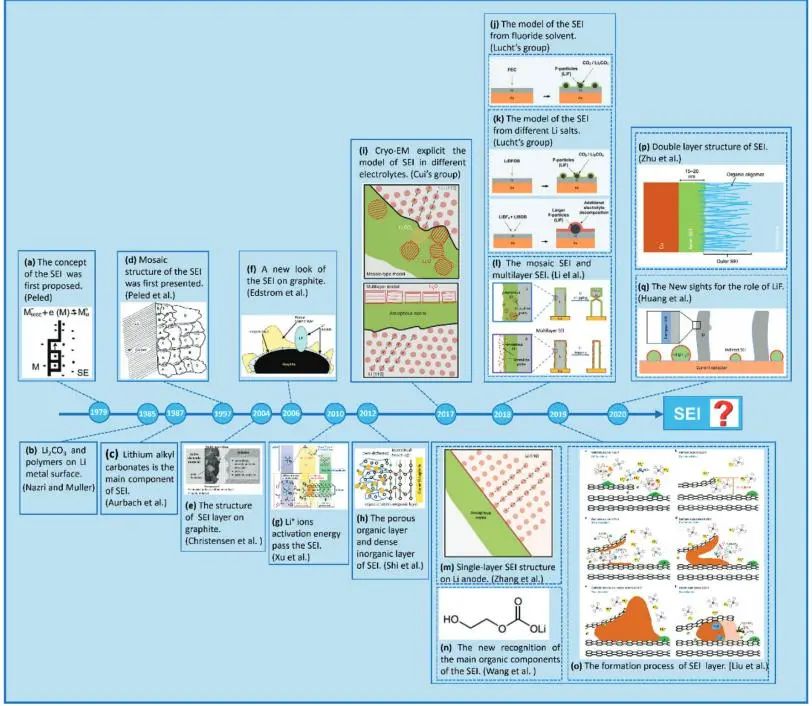
Figure 1. Research progress and development time of SEI on the surface of graphite and lithium metal ¹
2. Experimental Equipment and Test Methods
2.1 Experimental Equipment
Model GVM2200(IEST), the test temperature range is 20°C~85°C, and it supports dual-channel (2 batteries) simultaneous testing. The appearance of the equipment is shown in Figure 2.

Figure 2. Appearance of GVM2200 Equipment
2.2 Test Information
2.2.1 NCM523/Graphite system battery cell, 0.5C CC to 4.2V, theoretical capacity 2400mAh

Figure 3. Test Cells
2.2.2 Test Method
Initially weigh the cell m0, put the cell to be tested into the corresponding channel of the device, open the MISG software, set the cell number and sampling frequency parameters corresponding to each channel, the software will automatically read the volume change, and test temperature, current, voltage, capacity and other data.
3. In-situ Volume Swelling Analysis of the Cell
Five parallel cells were formed under the conditions of 25°C, 45°C, 55°C, 65°C and 85°C according to the process shown in Figure 4(a), and the volumes shown in Figure 4(b) and (c) were obtained swelling curve and differential capacity curve. As the formation temperature increases, the corresponding gas production also gradually increases, and when the battery is charged to about 3.7V, the volume curve of the battery reaches a relatively stable maximum value, and the volume shrinks slightly in the constant voltage stage. From the enlarged volume swelling curve and differential capacity curve, the increase of the formation temperature will cause the volume swelling to occur earlier, and the peak positions of each phase transition will shift to the left, which shows that the polarization of the battery is continuously decreasing, but when the temperature reaches above 55 °C When , the peak of the first phase transition reaction is sharper, which may be related to the more intense reaction of the SEI film formed at high temperature.
During the formation process, the surface of the graphite electrode forms a solid electrolyte interface(SEI) to prevent solvent co-intercalation. The physical and chemical properties of the interface can significantly affect the polarization potential and lifetime of Li-ion batteries. An ideal SEI layer requires high ionic conductivity, good electronic insulation, and good thermal and electrochemical stability to ensure the rapid transport of lithium ions and the side effects of sealing and isolating electrons. The main group of SEI will include electrolyte salt and LiF, Li2CO3, RCO2Li, carbonate, etc. Only when a stable SEI is successfully formed, lithium ions can be stably intercalated and deintercalated with graphite. The capacity retention and storage life of Li-ion batteries also directly depend on the stability of SEI.
The formation of SEI has two reverse processes: The increase of SEI growth and the decrease of SEI dissolution. Studies have shown that SEI growth is related to the electrochemically induced reduction process of the electrolytic solvent and is less sensitive to temperature. On the contrary, the increase of temperature greatly accelerated the dissolution of the initially formed SEI into the electrolyte. Therefore, the SEI interface formed at different temperatures has different characteristics. At high temperatures, both solvent molecules and electrodes are relatively active, and the electrochemical performance of the electrode/electrolyte interface becomes more complicated. The organic components of the SEI are more easily dissolved in the organic electrolyte than the inorganic components, leading to the collapse of the SEI film. Therefore, the inorganic component becomes the main component of the SEI film at high temperature, and the ability of the electrode to withstand volumetric deformation is significantly reduced. High temperature will also cause serious side reactions and generate more gas; moreover, at high temperature, the transmission speed of lithium-ion becomes faster, and the electrochemical stress of the interface is greater, which will also lead to interface instability.
At low temperature, the formed SEI will be denser, resulting in lower ionic conductivity, which limits the rapid transport of Li, and if the temperature is too low due to high polarization, it will also lead to the direct deposition of metallic lithium. Therefore, only in the appropriate temperature range, the formed interfacial film has the best ionic conductivity and stability. Anyway,the formation temperature will change the viscosity and conductivity of the electrolyte and the ion diffusion speed of the electrode material, thereby affecting the formation effect. Generally, the higher the formation temperature, the lower the viscosity of the electrolyte, the higher the conductivity of the electrolyte, and the faster the ion diffusion rate of the electrode material, so the higher the temperature, the smaller the polarization of the battery and the better the formation effect. However, an excessively high formation temperature will destroy the structure of the formed SEI film, increase side reactions, and accelerate the volatilization of low-boiling components in the electrolyte, which is not conducive to the formation effect 2. Therefore, the most selected formation temperature in the industry is 45~70°C.
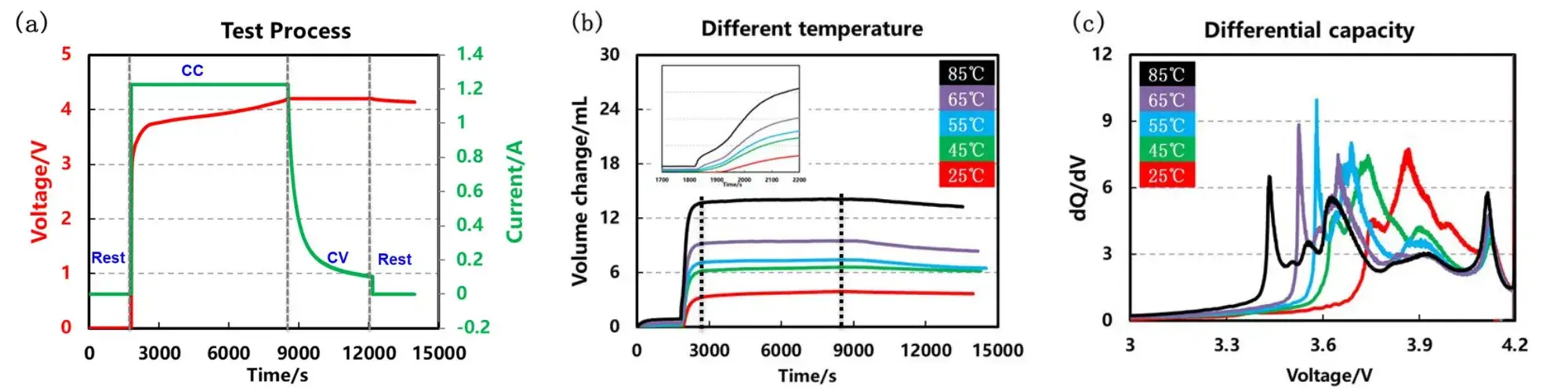
Figure 4. Cell formation process, volume swelling and differential capacity curves
4. Summary
In this paper, a temperature-controllable dual-channel in-situ gas production volume monitor is used to conduct in-situ volume swelling tests under different formation temperatures. It is found that the higher the temperature, the earlier and larger the cell volume swelling. Quantitative characterization of cell volume can help battery developers determine optimal formation conditions.
5. References
[1] Jian Tan, John Matz, Pei Dong, Jianfeng Shen, and Mingxin Ye. A Growing Appreciation for the Role of LiF in the Solid Electrolyte Interphase. Adv. Energy Mater. 2021.DOI:10.1002/aenm.202100046
[2] Wei Wenfei, Zhong Kuan, Jiang Shiyong. Effect of the formation temperature on lithium-ion battery performance[J]. Energy Storage Science and Technology, 2018, 7(5): 908-912.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


