-
iestinstrument
Cell Overcharge In-situ Gas Production Analysis Of Cathode Materials And Electrolytes
1. Preface
In this paper, an In-Situ Gassing Volume Analyzer(GVM) was used to perform in-situ overcharge volume test on NCM523/graphite cells (theoretical capacity 1000mAh) with different additive types and contents, and analyze the gas production behavior of the cells.
During the overcharging process of lithium ion batteries, a series of chemical and electrochemical reactions will occur, including the reversible phase transition of the cathode and anode materials, irreversible structural changes, and the oxidation reaction of electrolyte components, etc, especially when the ternary cathode material is unstable under high voltage and lattice oxygen is precipitated, it will further cause the oxidation reaction of the electrolyte components to produce gas, which will cause the swelling of the cell. Figure 1 is the use of OEMS in-situ monitoring equipment to monitor the gas composition changes of the NCM811 cycle overcharge 1.
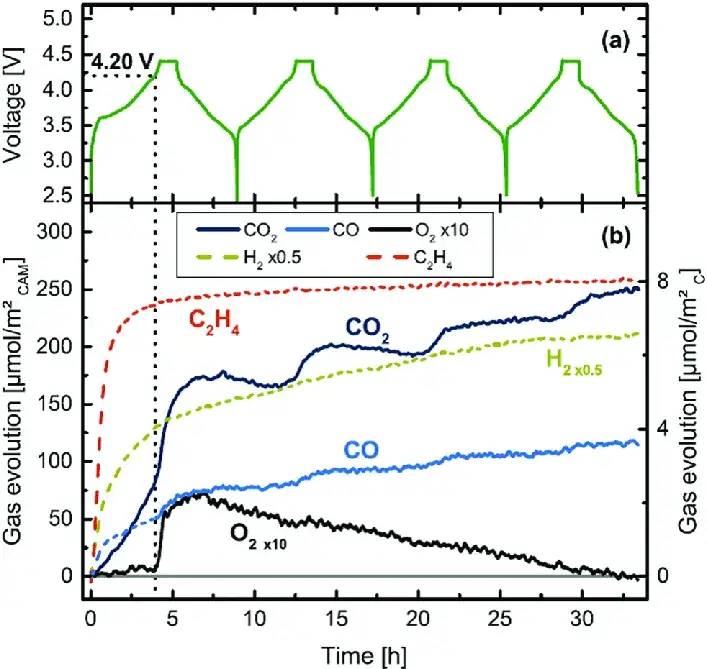
Figure 1. NCM811 In-situ gas component monitoring1
2. Experimental Equipment and Test Methods
2.1 Experimental Equipment
Model GVM2200(IEST),test temperature range 20~85℃,support dual-channel (2 cells) synchronous test,the appearance of the equipment is shown in Figure 2.
Figure 2. The Appearance of GVM2200
2.2 Test Parameters
25℃ 1C CC to 5V.
2.3 Test Method
Initially weigh the cell m0, put the cell to be tested into the corresponding channel of the device, open the MISG software, set the cell number and sampling frequency parameters corresponding to each channel, the software automatically reads the volume change, test temperature, current, voltage , capacity and other data.
3. Gas Production Analysis of Cell Overcharge In-situ
3.1 Charge-discharge Curve and Volume Change Curve Analysis
The volume change curve and voltage curve of the cell are shown in Figure 3(a)(b)(c),C1 and C2 are two kinds of cathode materials with different electrolyte additives are type E1 and E2, and the content of the additive:0%、1%、2%、3%、5%. Comparing Figures 3(a) and 3(b), are matched with the same electrolyte, as the electrolyte additive content increases, the volume change of the two groups of cells becomes larger and larger,it is explained that the gas production reaction of the additive causes the cell to swell. Further comparing the total gas production of the cells, it can be found that the cell corresponding to the C2 cathode material produces more gas. This may be due to the more unstable structure of the material at high voltages, releasing more lattice oxygen to react with the electrolyte. Comparing Figures 3(a) and 3(c), the same cathode material with different electrolytes, the volume of the cell still increases with the increase of the additive content, but the total gas production of the two groups of cells is almost the same, it shows that the type of additive does not affect the total gas production. In addition, It can be seen from the three sets of data that when the content of additives reaches 5%,it can be seen from the voltage curve that it is difficult for the voltage of the cell to reach the upper limit of 5V , this may be due to the fact that the cell produces more gas, which leads to the deterioration of the interface contact between the electrodes and the greater polarization of the cell.
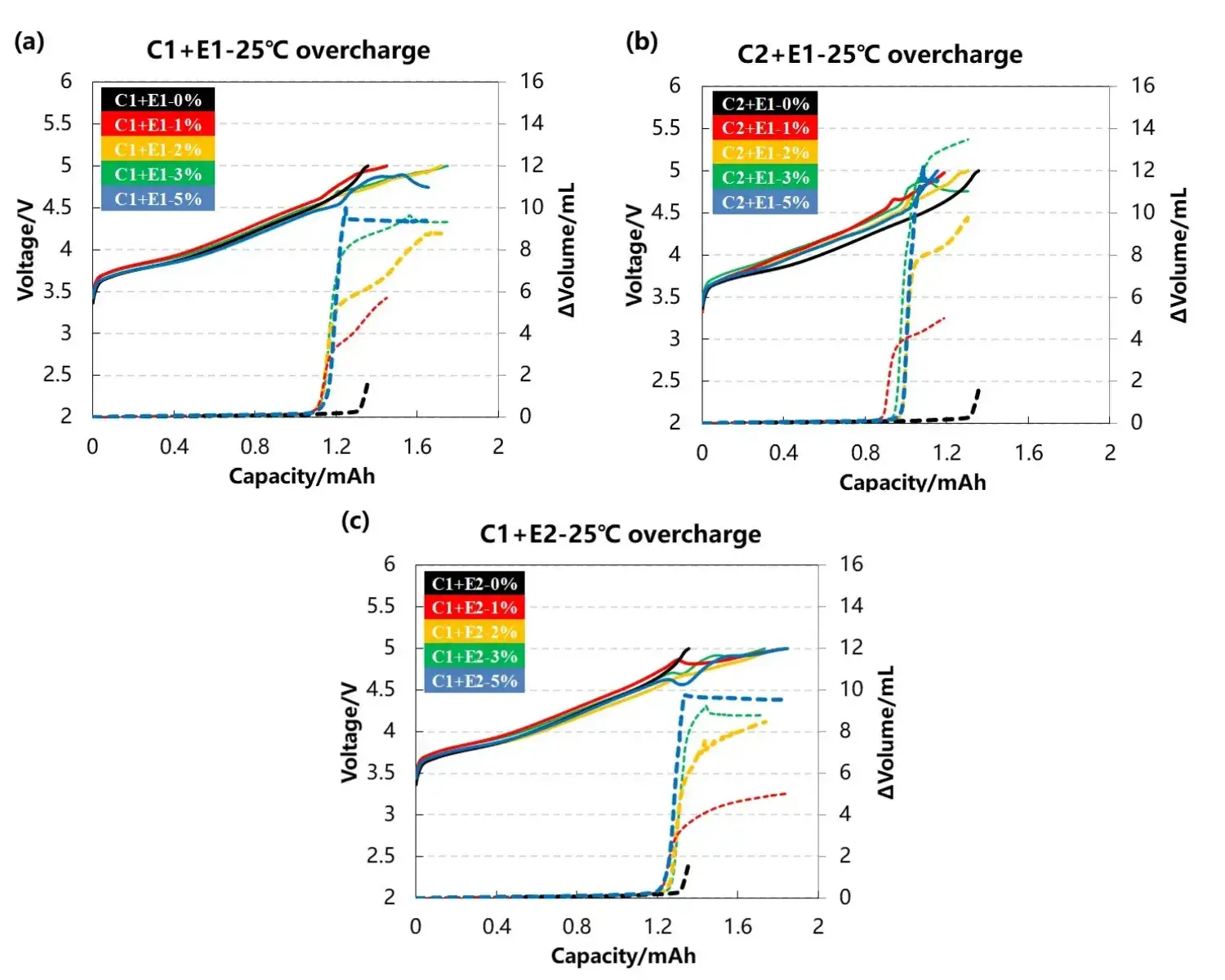
Figure 3. Cell voltage and gas production curves of different cathode materials with different electrolytes
2.2 Analysis of Gas Production Capacity and Gas Voltage of Cells
The total gas production and the inflection point voltage information of the gas production curve of the three groups of cells are shown in Table 1 and Figure 4. With the increase of the additive content, the total gas production of the cell corresponding to the E1 and E2 additive types are gradually increasing, and when used with the C2 cathode material, the total gas production of the cell will be significantly higher. Comparing the same additive, as the content increases from 1% to 5%, the initial voltage of the cell’s gas production changes less. Therefore, the type of cathode material and the electrolyte additive content will affect the total gas production of the cell, and the type of additive will affect the gas production potential of the cell,choosing the right cathode material, the type and content of additives can control the overcharging and gas production behavior of the cell.
Table 1. Information about gas production and gas production potential of cells with different cathode materials and different electrolytes
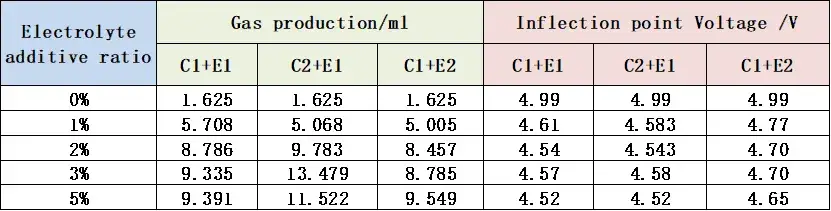
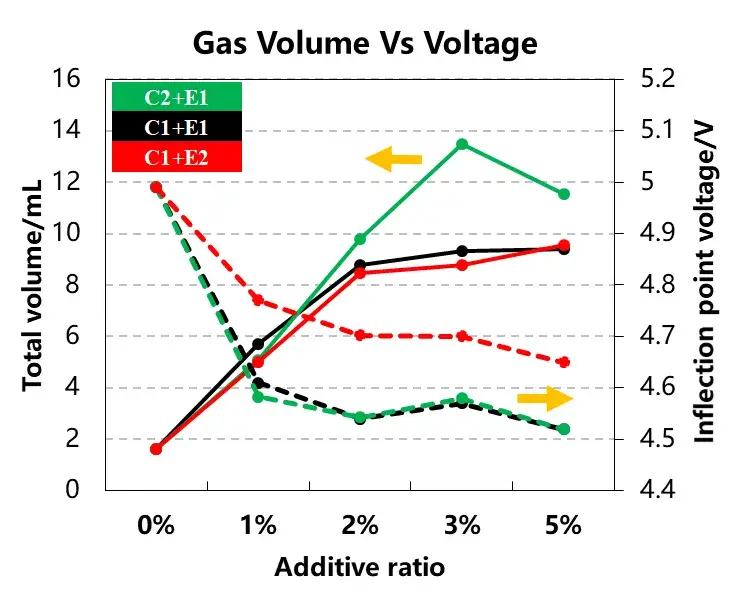
Figure 4. Gas production volume and gas voltage curve of different cathode materials and different electrolytes
3. Summary
In this paper, a temperature-controllable dual-channel In-Situ Gassing Volume Analyzer(GVM) is used to compare and analyze the overcharge and gas generation behavior of lithium-ion batteries when matched with different cathode materials and different electrolytes. It can be found that the cathode material type and electrolyte additive content will affect the total gas production of the cell, and the additive type will affect the gas production potential of the cell. Therefore, selecting the appropriate cathode material, electrolyte additive type and content can control the overcharge and gas generation behavior of the cell.
4. References
[1] Roland Jung et al. Oxygen release and its effect on the cycling stability of LiNixMnyCo2O2(NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 2017, 164 A1361.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



