-
iestinstrument
Research Status And Conductivity Evaluation Of LMFP Materials
1. Research Background
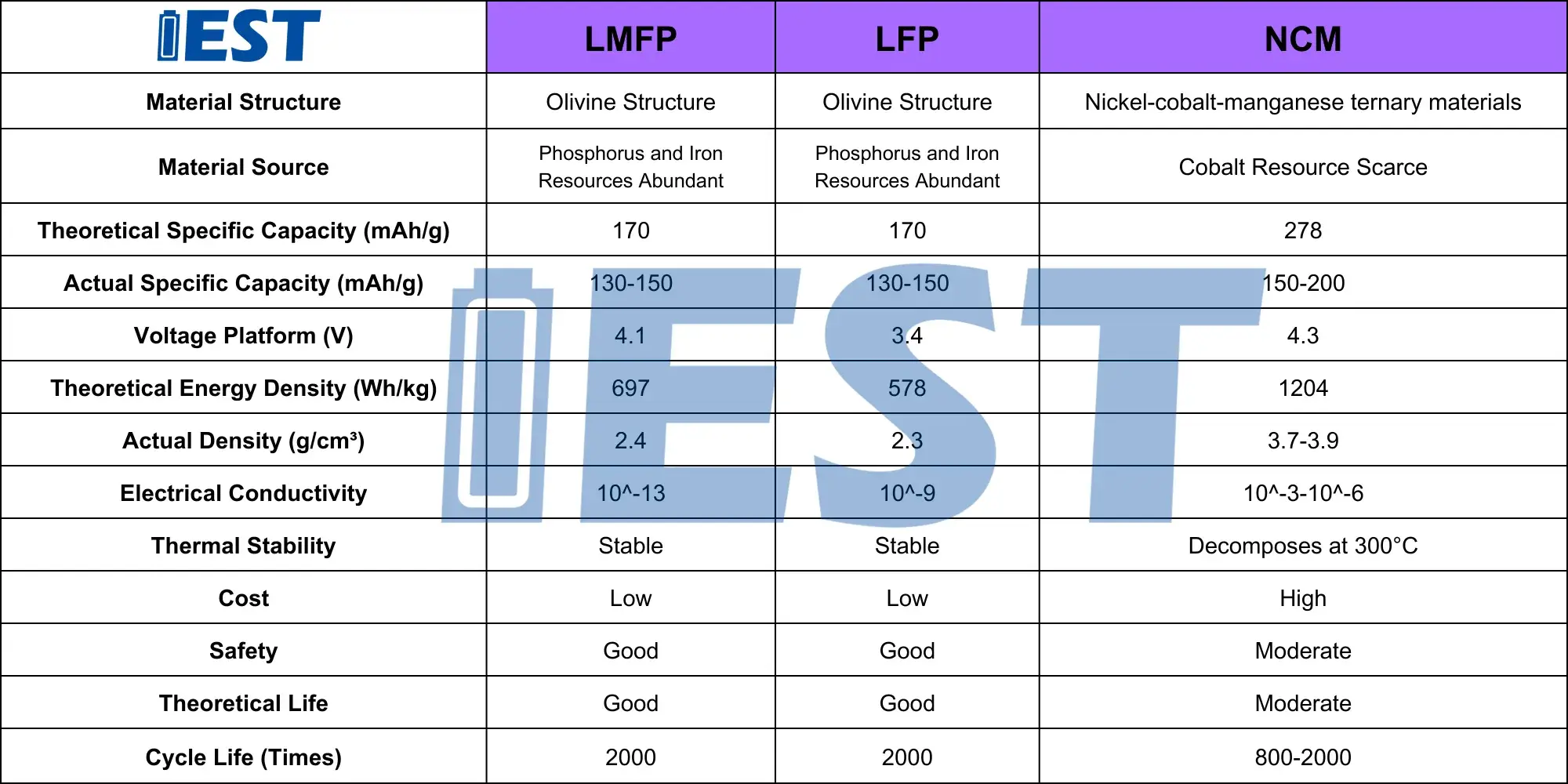
At present, the cathode materials of lithium-ion batteries are mainly lithium cobalt oxide (LCO), ternary materials (NCM) and lithium iron phosphate (LFP). In the past one or two years, with the increase of electric vehicles and the gradual expansion of energy storage, people’s attention to battery safety has also gradually increased. In addition to the improvement of battery design technology (such as BYD’s blade battery, CATL’s CTP technology, etc.), the research and development of new materials is also particularly critical. LFP has gradually become the main cathode material for electric vehicles or energy storage batteries due to its high safety. However, for LFP batteries, the development of its energy density has almost reached its limit, and there is not much room for performance improvement. The crystal structure of lithium manganese iron phosphate (LMFP material) is similar to that of LFP, and it also has the characteristics of stable chemical properties and excellent safety performance. At the same time, the manganese element doped in LMFP material can increase the charging voltage of the material, increasing the charging voltage from 3.4V of LFP to 4.1V, which increases the theoretical energy density of LMFP batteries by 15~20%, further extending the driving range. LMFP material has better safety performance than NCM material and higher energy density than LFP material. In addition, LMFP materials has low dependence on rare metals and can be produced on the same line with LFP, with obvious cost advantages. The detailed performance comparison of LMFP and other cathode electrode materials is shown in Table 1.
Table 1. Comparison of LMFP materials with other cathode materials
2. Process Route
The synthesis method of LMFP materials and LFP materials are basically the same, which is mainly high temperature solid-phase method, hydrothermal synthesis method, co-precipitation method, etc. In the industry, there is no uniform standard for the preparation process of LMFP. At present, there is no unified standard for the preparation process route of LMFP, and the technical routes of leading manufacturers in the industry are as follows:
(1) Shenzhen Dynanonic Co: mainly adopts sol-gel method to prepare LMFP, the lithium source, manganese source, phosphorus source and iron source are mixed and dissolved proportionally to get the liquid slurry, which is removed from the water and crushed to get the powder precursor, and then sintered and crushed to get the LMFP material.
(2)Lithitech: mainly adopts the co-precipitation method to get the precursor containing iron and manganese by co-precipitation method, and then the precursor and lithium source and carbon source are evenly mixed to get the LMFP material after sintering.
(3) CATL: the main use of solvent heat preparation of LMFP material, the required raw materials will be dissolved in the solvent, configured into a uniform solution, which will be transferred to the reaction kettle to obtain the precursor, and then drying, sintering to obtain LMFP material.
(4) Skylandone: It is mainly synthesized by high-temperature solid-phase method, and the required raw materials are mixed uniformly, and then high-temperature sintering is carried out to obtain LMFP material, and compounded with ternary materials for supply.
3. Material Modification
The one-dimensional conduction of lithium ions in olivine-type cathode materials determines their low ionic conductivity, and in terms of electron transfer capability, LMFP has a lower conductivity than lithium iron phosphate with semiconducting properties, with lithium iron phosphate having a conductivity of 10-9 S/cm, NCM having a conductivity of 10-3 S/cm, and LMFP having a conductivity of 10-13 S/cm only.Structurally, LMFP has no continuous FeO6 (MnO6) coplanar octahedral network, but through the PO4 tetrahedral connection (as shown in Figure 1), so it can not form a continuous Co-O-Co structure like lithium cobaltate material, which restricts the movement of lithium in a one-dimensional channel, resulting in poor conductivity of the material, which leads to the poor performance of the large multiplication of charging and discharging performance is also very poor. The improvement of electrical conductivity is mainly focused on carbon coating and ion doping. Carbon coating mainly improves the electronic conductivity, while ion doping mainly improves the ion diffusion coefficient and conductivity.
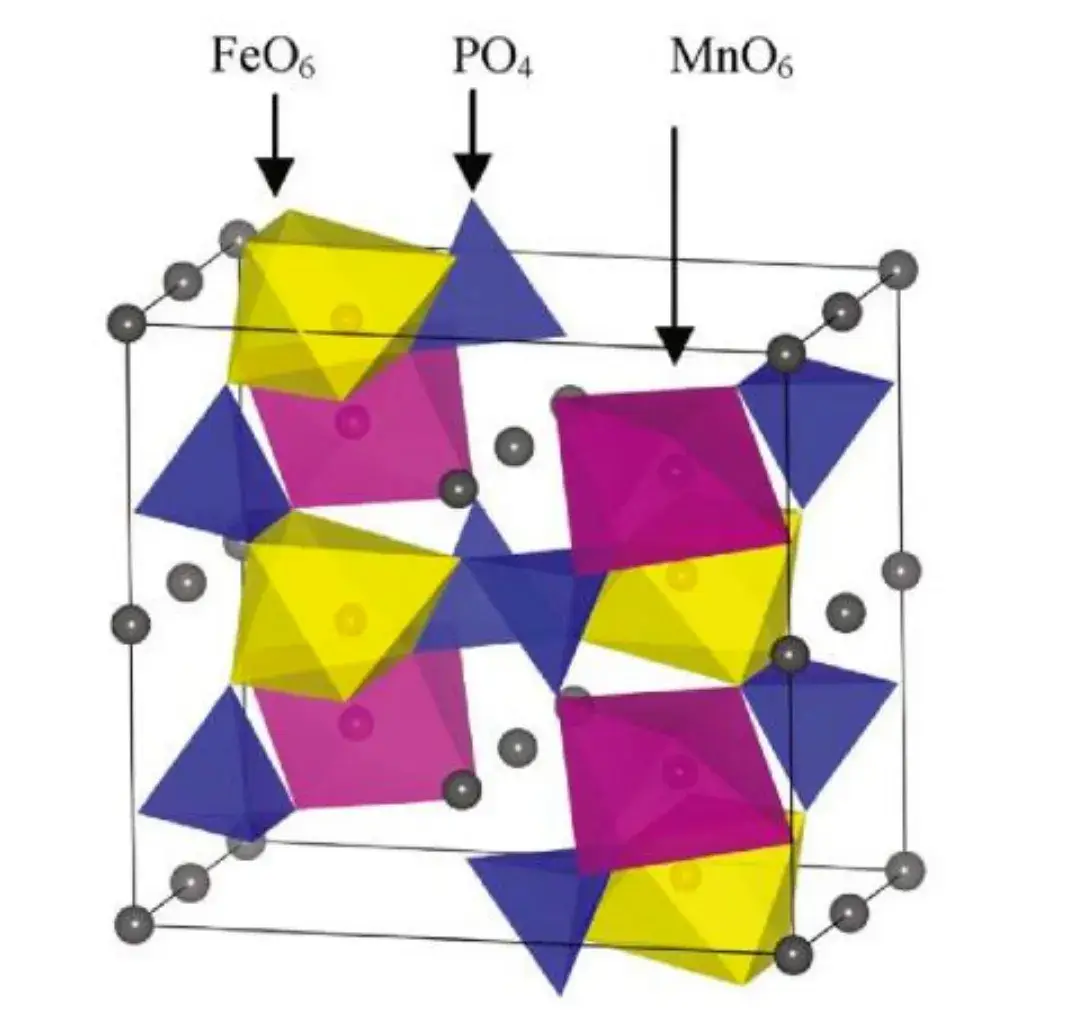
Figure 1. Schematic structure of olivine type LMFP[1]
Adding an appropriate amount of carbon in the material synthesis process can not only improve the conductivity of the material, but also prevent the contact between particles and inhibit the agglomeration and growth of particles, so it is easier to obtain nano-sized cathode materials[2, 3], which effectively reduces the diffusion distance of Li inside the active particles and enables the material to have a more excellent multiplicity. At the same time, carbon coating can also reduce the contact surface between the active material and the electrolyte, thus avoiding the side reaction with the electrolyte and improving its high temperature performance and cycling performance. Carbon coating is generally divided into two types: one is to mix the finished LMFP product with the carbon source, and then calcine the coating at a high temperature under a reducing atmosphere; the other is to add the carbon source directly into the raw material, and then mixing, drying, and high-temperature sintering together to form the carbon-covered LMFP/C composite material. For example, Oh and other researchers used ultrasonic dispersion pyrolysis to synthesize LiMnx Fe1-x PO4 powder[4], and then ball-milled and mixed with the carbon source to obtain carbon-covered olivine-type lithium-ion cathode materials. The first discharge specific capacities of the material were 150 mAh/g and 121 mAh/g when discharged at 0.5 C and 2 C multiplicity, respectively, and the electrochemical performance was improved thanks to the close bonding of primary particles with carbon and the uniform coating of carbon.
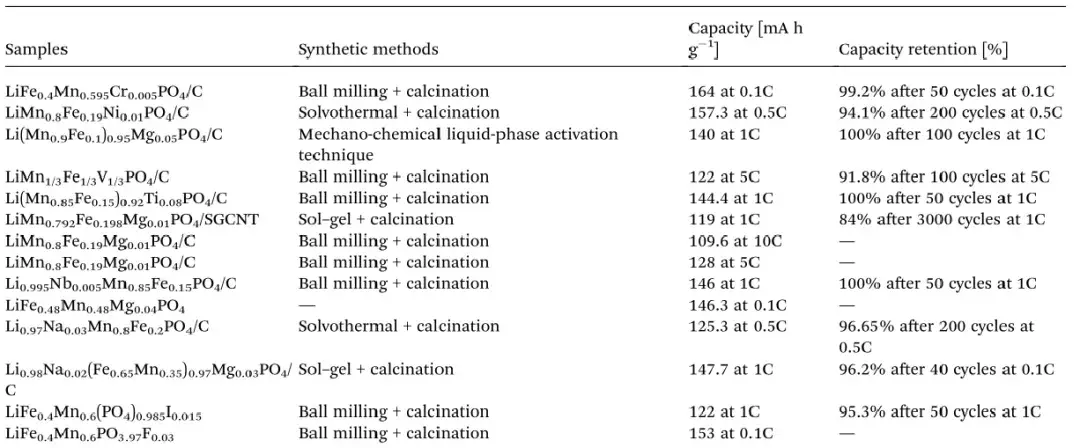
In addition to coating carbon on the material surface to improve the electronic conductivity of the material, ion doping is also a common means to improve the lithium diffusion coefficient and ionic conductivity. Ion doping can form Li-site or Fe and Mn-site defects in the LMFP lattice, create vacancies in the material lattice or change the inter-atomic bond lengths, which facilitates the movement of Li+ in the lattice and thus improves the electrochemical properties of the material[5]. Compared with modification methods such as morphology control and surface coating, the advantage of ion doping is that it can increase the bulk energy density with less effect on the vibrational density of the LMFP material, which is conducive to the enhancement of the multiplicity performance of the battery. Table 2 demonstrates a summary of data on elemental doping modification of LMFP material in recent years.
Table 2. Summary of data on the effect of different elemental doping on LMFP material performance[6]
4. Conductivity Test Methods
The various methods introduced above can effectively improve the conductivity and electrochemical performance of cathode materials. In terms of performance characterization, researchers usually test the electrochemical performance by assembling button batteries or pouch cells, or characterize the impedance change by testing EIS to judge the effect of modification, so how to accurately and quickly test the conductivity change before and after material modification? According to Ohm’s law R=U/I, the resistance of a conductor can be calculated by testing the current through the conductor and the voltage drop through the conductor, and combined with the geometric dimensions of the sample to be tested, the conductivity can be calculated by the following formula.
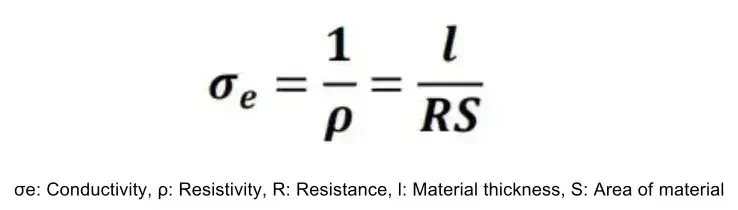
Figure 2. Conductivity calculation formula
The test method we generally called DC method, the electrode material is a mixture of ions and electrons conductor, the test process through the DC polarization, the mixture of ions and electrons of the high transient current will soon drop, and finally reach a stable electronic current, so as to mainly determine the electronic conductivity. DC method also includes two-probe method and four-probe method, IEST through a large number of test experiments found that: the two-probe principle is more suitable for samples with slightly higher resistance, such as LCO and low nickel NCM lithium anode materials, while the four-probe principle is more suitable for samples with smaller resistance, such as graphite anode and various types of conductive agents, etc.; and the resistance value of the samples in the ohmic level of the carbon cased LMFP, LFP, etc., the two principles are equally applicable. The two principles are equally suitable for samples with ohmic resistance such as carbon coated LMFP, LFP, etc. Comparative tests have shown that there is not much difference between the test results of the two principles. For this reason, IEST has independently developed a dual-principle, dual-function device for the determination of large and small resistance samples – Powder Resistivity & Compaction Density Tester (PRCD3100, IEST). During the testing process, while applying different pressures (up to 5T) to the powder samples, the device can synchronously collect parameters such as resistance, resistivity, conductivity, compaction density, etc. of the powder samples and display them on the software interface in real time.
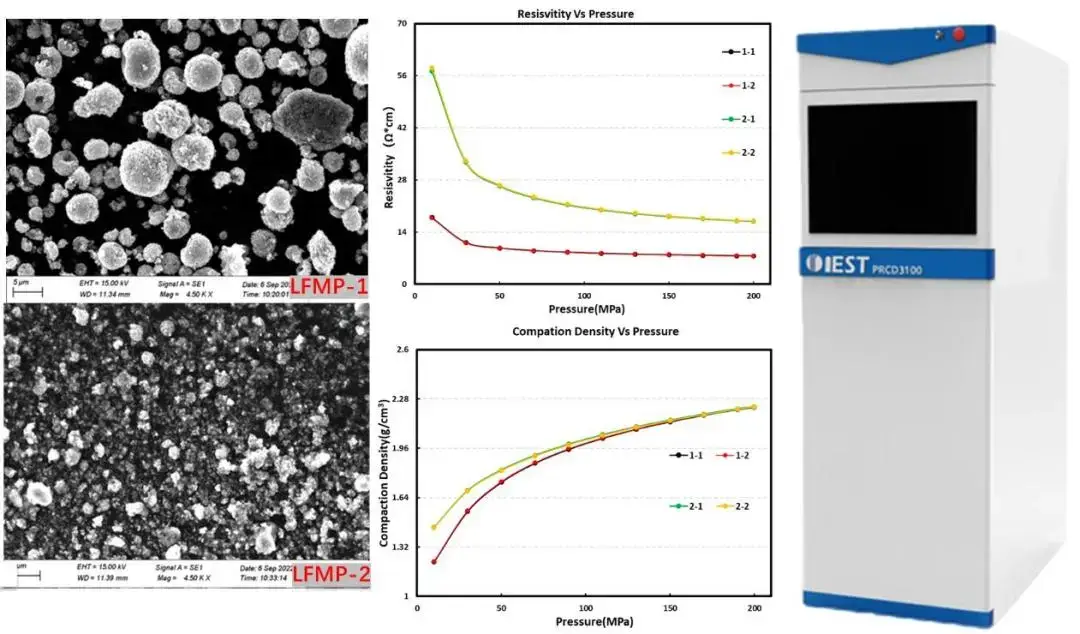
Figure 3. Schematic diagram of Powder Resistivity & Compaction Density Tester(PRCD3100) instrument and different LFMP material test data
5. References
[1] Osorio-Guillén J M,Holm B,Ahuja R,et al. A theoretical study of olivine LiMPO4 cathodes[J]. Solid State Ionics, 2004, 167(3-4): 221-227.
[2] Wang Y, Hu G, Cao Y, et al. Highly atom-economical and environmentally friendly synthesis of LiMn0.8Fe0.2PO4/rGO/C cathode material for lithium-ion batteries[J]. Electrochimica Acta, 2020,354:136743.
[3] Kosova N V, Podgornova O A, Gutakovskii A K. Different electrochemical responses of LiFe0.5Mn0.5PO4 prepared by mechanochemical and solvothermal methods[J]. Journal of Alloys and Compounds, 2018, 742: 454-465.
[4] Oh S M, Jung H G, Yoon C S, et al. Enhanced electrochemical performance of carbon-LiMn1−x FexPO4 nanocomposite cathode for lithium-ion batteries[J]. Journal of Power Sources, 2011, 196(16): 6924-6928.
[5] Budumuru A K, Viji M, Jena A, et al. Mn substitution controlled Li-diffusion in single crystalline nanotubular LiFePO4 high rate-capability cathodes: Experimental and theoretical studies[J]. Journal of Power Sources, 2018, 406: 50-62.
[6] Yang L , Deng W , Xu W ,et al. Olivine LiMnxFe1-xPO4 cathode materials for lithium ion batteries: restricted factors of rate performances[J].Journal of Materials Chemistry A, 2021, 9: 14214–14232.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


