-
iestinstrument
Impact of High-temperature Storage at Different SOCs on Gas Generation in Battery Cells
1. Background
Lithium-ion batteries with high specific energy, long life and other advantages have been widely used in consumer electronics, electric vehicles and energy storage. Different application scenarios have different degrees of requirements for high temperature storage, especially in the field of cell phones, tablet and laptop have clear requirements for high temperature storage of lithium batteries. At present, some technicians have already studied the impact of different temperature storage at different voltages on the performance of the battery core, and have also explained the corresponding mechanism. However, in-situ quantitative analysis of the volume change of high-temperature storage cells has rarely been reported. In this paper, we mainly use the in-situ gas generation volume monitor (GVM2200) of IEST to comparatively monitor the open-circuit voltage and volume change of different SOC batteries during high-temperature storage.
2. Test Information
2.1 Test Equipment
In-situ gassing volume monitor, model GVM2200 (IEST), adjustable temperature 20°C~85°C. Figure 1 shows the appearance of the device.
Figure 1. Schematic diagram of the in-situ gassing volume monitor(GVM2200)
2.2 Test Parameters
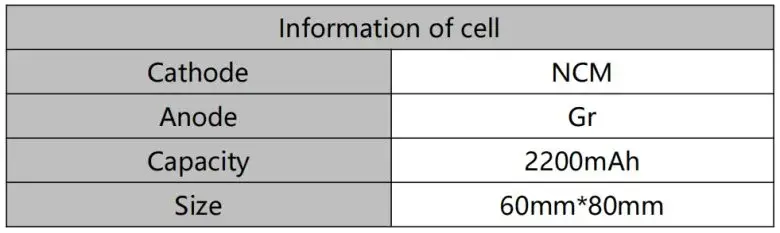
The battery cell information is shown in Table 1.
Table 1. Battery Cell Information
3. Result analysis
Select five parallel sample cells, and adjust their SOC at room temperature to 100%, 80%, 50%, 30%, and 0%. After the adjustment, the cells were left for 15 hours, and then the in-situ volume monitor (GVM2200) was started to record the voltage and volume of different SOC cells in an 85°C oil bath over time.
3.1 Voltage Change
As shown in Figure 2: the graph on the left shows the change curve of the open-circuit voltage stored at 85°C for 7 days, and the graph on the right shows the change curve of the open-circuit voltage after 1 hour of testing. It can be seen that with the prolongation of storage time, the overall open circuit voltage shows a downward trend, and with the decrease of the storage SOC of the battery cell, the downward trend continues to slow down. The initial test cell was put into an 85°C oil bath environment from room temperature, there was a thermal equilibrium process, and the open circuit voltage change within the first hour was compared and analyzed (right figure): 100% and 80% SOC group cells showed a downward trend, 50% %, 30% and 0% batteries have a rising stage. This is related to the entropy heat coefficient of the battery core: from the definition of Gibbs free energy and enthalpy, and then through the corresponding mathematical operations, it can be obtained.
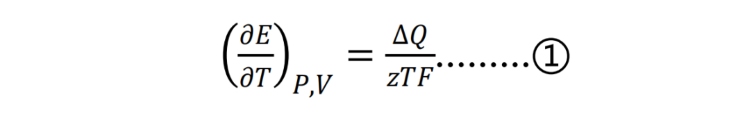
Among them, E represents the open circuit voltage, z represents the transfer quantity of electrons in the chemical reaction equation, which is always positive; T represents the absolute temperature, which is always positive; F is Faraday’s constant, which is always positive; ∆Q represents the heat of reaction of the battery under a specific SOC, which can be positive or negative. The battery entropy change coefficient ∂E/∂T is an important physical parameter that characterizes the thermal characteristics of the battery. It represents the change of the battery electromotive force with temperature, and can reflect the reversible heat generation of the battery during charging and discharging; When the entropy change coefficient is a negative value, the current is a negative value during the discharge process, the reversible entropy of the battery becomes a positive value, and the reversible heat of the battery is exothermic; When the entropy change coefficient is a positive value, the reversible entropy of the battery becomes negative during the discharge process, and the reversible heat of the battery is shown as heat absorption. Combining the formula ① and the initial voltage change, it can be seen that when the cell is at 100% and 80% SOC, the cell is placed in an oil bath environment of 85°C from room temperature, and the potential decreases as the temperature increases, the entropy change coefficient ∂E/∂T is negative, and the discharge process is an exothermic reaction at this time; at 0%, 30%, and 50% SOC, the potential increases with the increase of temperature, the entropy change coefficient ∂E/∂T is positive, and the discharge process is an endothermic reaction. That is to say, the thermal effect of the battery will change with the change of SOC during the charging and discharging process. When the battery is in a low state of charge (0%-50%SOC), the lithium ions inside the battery are embedded in the positive electrode material and enriched around the positive electrode. When the temperature rises, the lithium ions inside the positive electrode material will be released from the positive electrode material under the action of heat, resulting in an increase in the battery potential, the battery voltage continues to increase, and the entropy heat coefficient becomes a positive value. When the battery is at a higher state of charge (80%-100% SOC), a large number of lithium ions are intercalated into the negative electrode material and enriched around the negative electrode, when the temperature of the battery rises, a part of lithium ions are released from the graphite negative electrode under the action of heat, and the potential of the negative electrode increases, so that the voltage of the battery as a whole decreases continuously, and the entropy change coefficient shows a negative value. During the whole process, the side reactions of the electrolyte also affect the thermal characteristics of the battery.
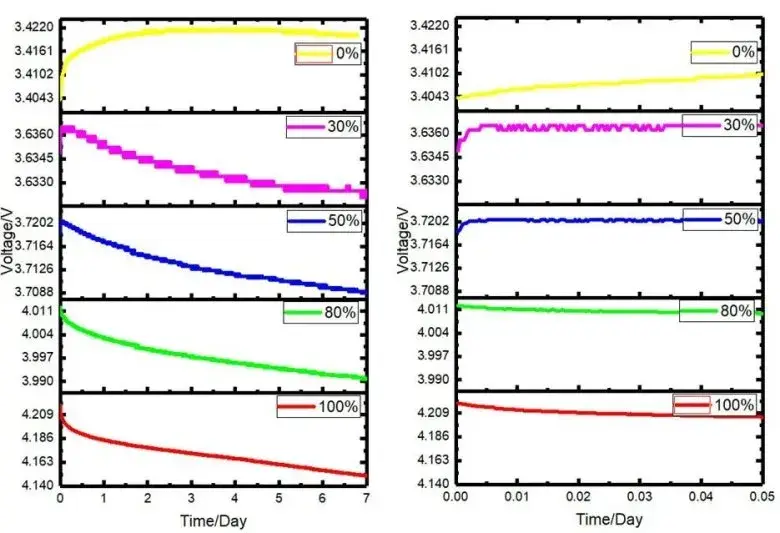
Figure 2. Curve of open circuit voltage versus storage time
3.2 Volume Change
GVM2200 in-situ monitors the gas generation of different SOC cells over time, as shown in Figure 3: the volume increase of cells stored at 85 degrees for 7 days is 20.3% (100% SOC), 10.9% (80% SOC), and 5.9% (50% SOC), 3.5% (30% SOC), 2.8% (0% SOC). The gas generation continues to increase as time goes on, and it shows a trend of increasing the total gas generation of the battery cells with the increase of the storage SOC. This gas generation behavior is mainly the result of the combined action of the electrolyte and the positive and negative electrodes. In the high SOC state, the positive electrode material with high potential is more likely to have a side reaction with the electrolyte to generate gas. At the same time, the SOC will also have a significant impact on the type of gas produced by the battery. Under a higher SoC, more types of gas will be produced. As the SOC decreases, the gas generation on the positive side of the cell gradually decreases, and the gas generation on the negative side gradually increases, and at low SOC, the gas generation on the negative side is much greater than that at high SOC, at the same time, the gas generation on the positive side is smaller than that on the negative side under low SOC cells¹, but the overall gas generation is less than that under high SOC conditions.
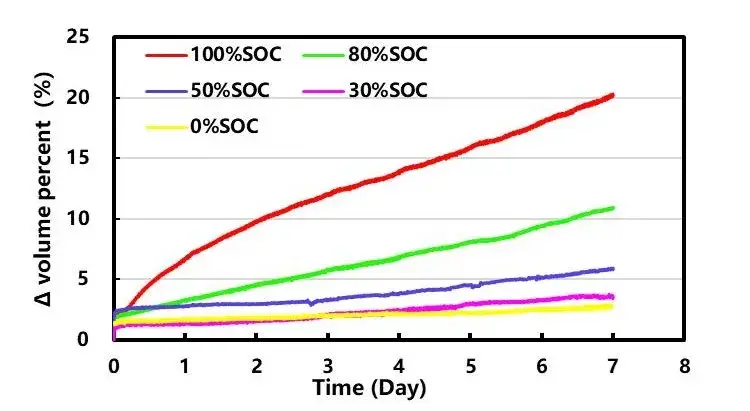
Figure 3. Gas generation versus time curve
3.3 Capacity Changes
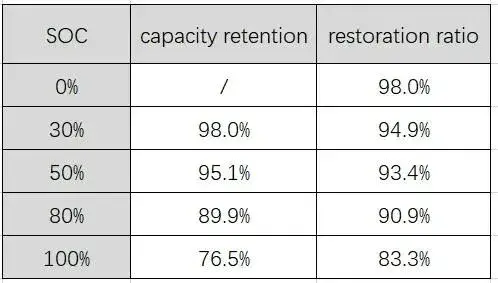
The capacity difference of each battery cell before and after storage is monitored separately as shown in the following table: the capacity retention rate decreases continuously with the increase of the storage SOC, and the capacity recovery rate also decreases continuously. The former is mainly due to the increase in SOC, which causes the cathode potential to increase and the oxidation to increase, while the anode potential decreases to increase the reductivity, both of which lead to an increase in the self-discharge rate of the battery, resulting in a continuous decline in capacity. The irreversible capacity loss may be due to some side reactions during high temperature storage. For example, these side reactions of the anode reducing the electrolyte will consume a part of the active lithium, and a large amount of products will be deposited on the anode. The inorganic components in the deposit hinder the diffusion of lithium ions, making the anode Reaction kinetic performance decreased [2] [3].
Table 2. List of capacity retention and recovery rates
4. Summary
In this paper, the in-situ gas generation volume monitor (GVM2200) is used to characterize the open circuit voltage and volume change of the battery cell during high temperature storage at 85°C, which can be used to guide us in voltage control during battery transportation, storage and work. It can also provide corresponding data support for accelerated aging simulation.
5. References
[1] Wang Nianju, Meng Fanhui, Yu Liwei, Zhou Jiang, Gao Jinhui, Effect of Voltage on Gas Production of Lithium-ion Batteries in High Temperature Storage[J], Power Technology, 2020. DOI: 10.3969/j.issn.1002-087X.2020.07.007.
[2] Yao Bin, Teng Guopeng, Liu Xiaomei, Chen Weifeng, Cai Yi. Mechanism of Lithium Iron Phosphate Battery High Temperature Storage Performance Attenuation[J]. Power Technology, 2018, (No. 7).
[3] Wei Zhiguo, Cheng Cheng, Yao Wangbing, etc., Effect of battery voltage on high temperature storage performance of lithium-ion batteries[J], Power Supply Technology, 2021. DOI: 10.3969/j.issn.1002-087X.2021.03.006.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



