-
iestinstrument
A Quasi-intercalation Reaction For Fast Sulfur Redox Kinetics In Solid-state Lithium-sulfur Battery
1. Article Abstract
In 2022, Dr. Li Chuang’s team from the Tsinghua Shenzhen International Graduate School developed a novel solid-state lithium sulfur battery (Li-SPAN) employing a salt-in-polymer solid electrolyte. This innovative design confines sulfur within a polyacrylonitrile (PAN) matrix during cycling. This confinement strategy effectively prevents the formation of insulating Li₂S, resulting in significantly faster sulfur redox kinetics and reduced electrode volume expansion compared to conventional solid-state lithium-sulfur systems. This work pioneers a new approach for enhancing kinetics by tuning the strength of the C-S bond, rather than relying on catalytic additives, offering a promising new direction for designing high-performance solid-state Li-S batteries.The analysis draws on experimental characterization (EIS, SEM, XPS, Raman, NMR), in-situ model coin-cell thickness monitoring (MCS1000), and full-cell cycling of 2032 coin and pouch cells.
2. Why this Lithium Sulfur Battery Architecture Matters
Lithium sulfur batteries promise high theoretical specific energy, but practical implementations typically suffer from severe volume change, active-material loss (polysulfide shuttling and Li₂S deposition), and sluggish sulfur redox kinetics. SPAN cathodes—sulfur chemically bound to a polyacrylonitrile backbone—mitigate many of these problems by immobilizing sulfur within a carbon-rich matrix. As a result, SPAN-based cells can show reduced conversion to insoluble Li₂S, smaller dimensional expansion during cycling, and improved reaction reversibility. These attributes make SPAN cathodes attractive for solid-state Li–S designs where mechanical stability and fast kinetics are essential.
3. Experimental Approach
The research methodology was structured as follows:
-
Material Preparation: The study utilized a 1PVHF1FSI solid electrolyte and a solid-state SPAN composite cathode.
-
Electrochemical Testing: Electrochemical Impedance Spectroscopy (EIS) measured the ionic conductivity of the electrolyte. Both 2032-type coin cells and pouch cells were assembled for evaluation.In-situ model coin cell swelling testing system(MCS1000) of the SPAN cathode and lithium anode was monitored using the MCS1000 measurement system (IEST Yuan Neng Technology).
-
Material Characterization: A suite of characterization techniques, including Scanning Electron Microscopy (SEM), X-ray Photoelectron Spectroscopy (XPS), Raman spectroscopy, and Nuclear Magnetic Resonance (NMR), was employed to analyze material properties and evolution.
4. Result Analysis
4.1 Performance of the Solid Polymer Electrolyte
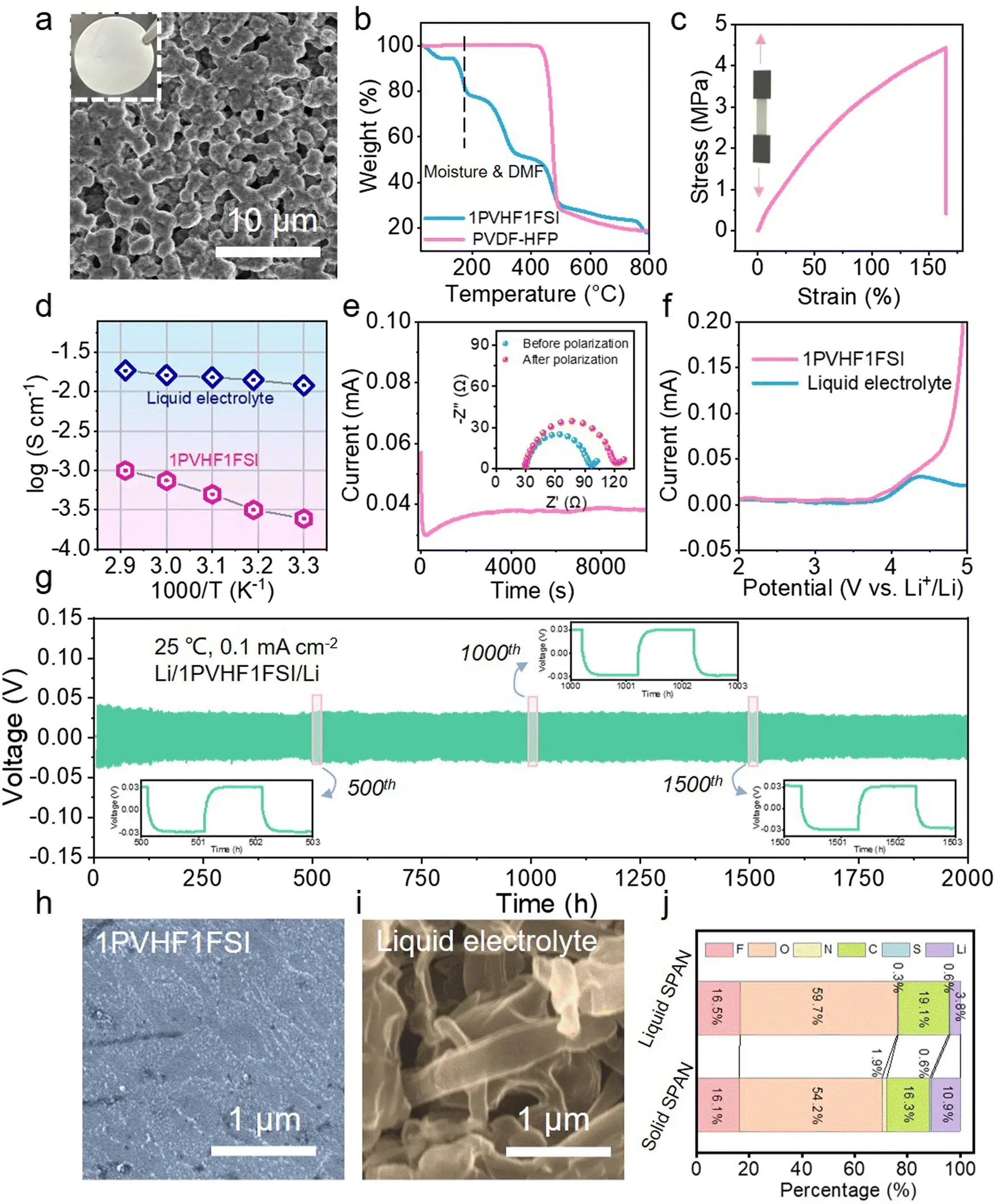
Figure 1. Performance characterization of solid electrolyte membrane 1PVHF1FSI in lithium sulfur battery
The author characterizes the performance of the solid electrolyte membrane 1PVHF1FSI in lithium sulfur battery in many aspects, it is found that it has a continuous porous channel, which can provide a good ion conduction path, and its good mechanical properties can inhibit the growth of lithium metal dendrites. In-situ thickness traces from the MCS1000 showed greatly reduced swelling of the SPAN cathode and lithium anode compared with typical conversion-based sulfur cathodes, consistent with a reduced net volume change during lithiation/delithiation.
The storage mechanism of lithium-ions in 1PVHF1FSI-based solid lithium sulfur battery is different from that in liquid lithium sulfur battery, subsequent authors characterized the polarization voltage, CV curve, cycle capacity, and rate performance of the three electrode materials, it is further clarified that solid-state SPAN has better cycle stability and rate capability due to high redox kinetics and low volume change.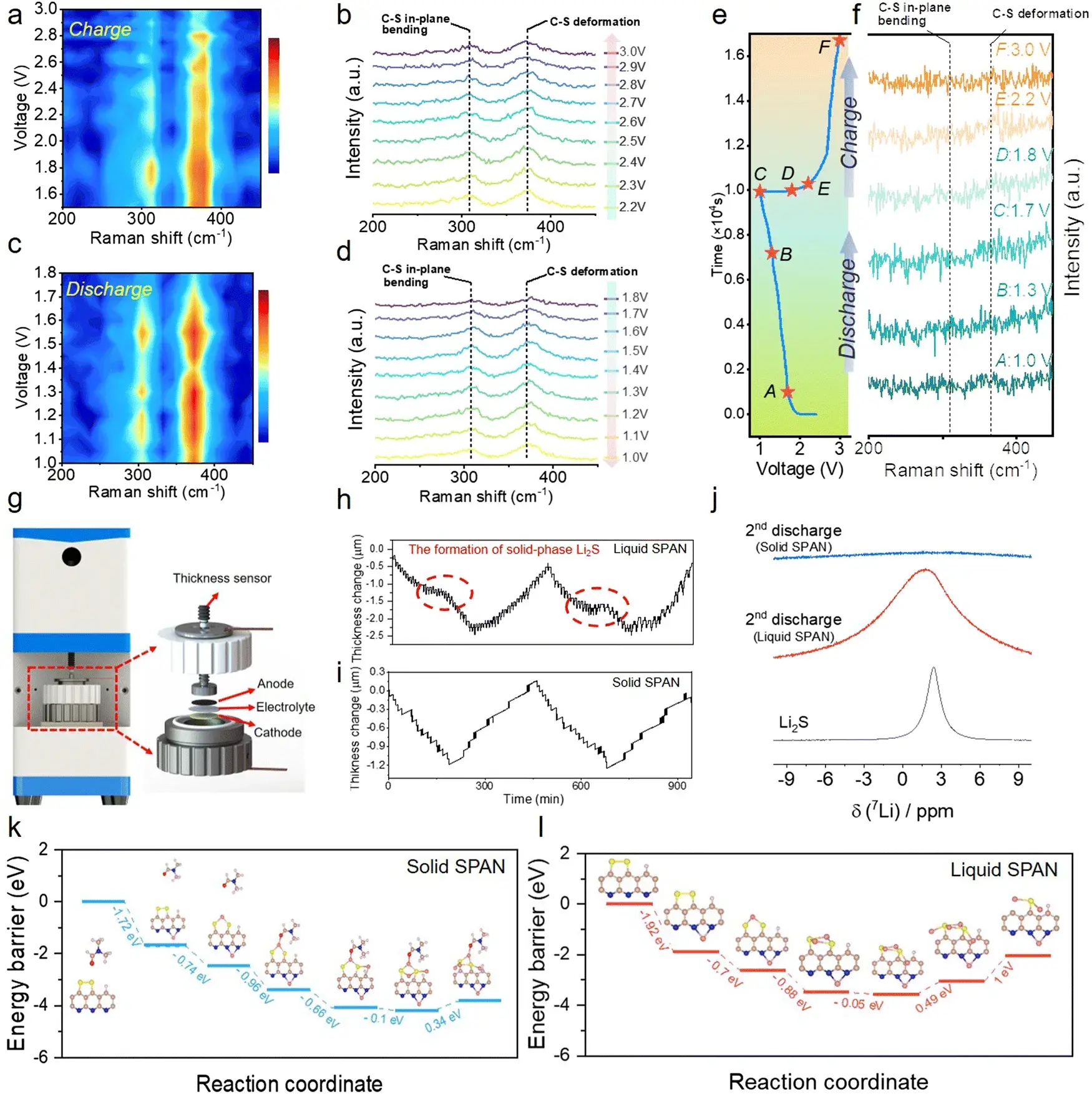
Figure 2. Analysis of the storage mechanism of Li in solid-state SPAN
4.2 Revealing the Lithium Storage Mechanism in Solid-State SPAN
To elucidate the storage mechanism, the team conducted in-situ Raman spectroscopy alongside in-situ expansion measurements. The results revealed a critical process: during lithiation, lithium-ion storage breaks the S-S bonds within the solid SPAN structure, leading to the formation of a Li₄S₂-PAN complex. This process resembles an insertion reaction rather than the typical conversion reaction seen in traditional Li-S chemistry. Consequently, the authors defined this novel mechanism as a “quasi-intercalation reaction.”
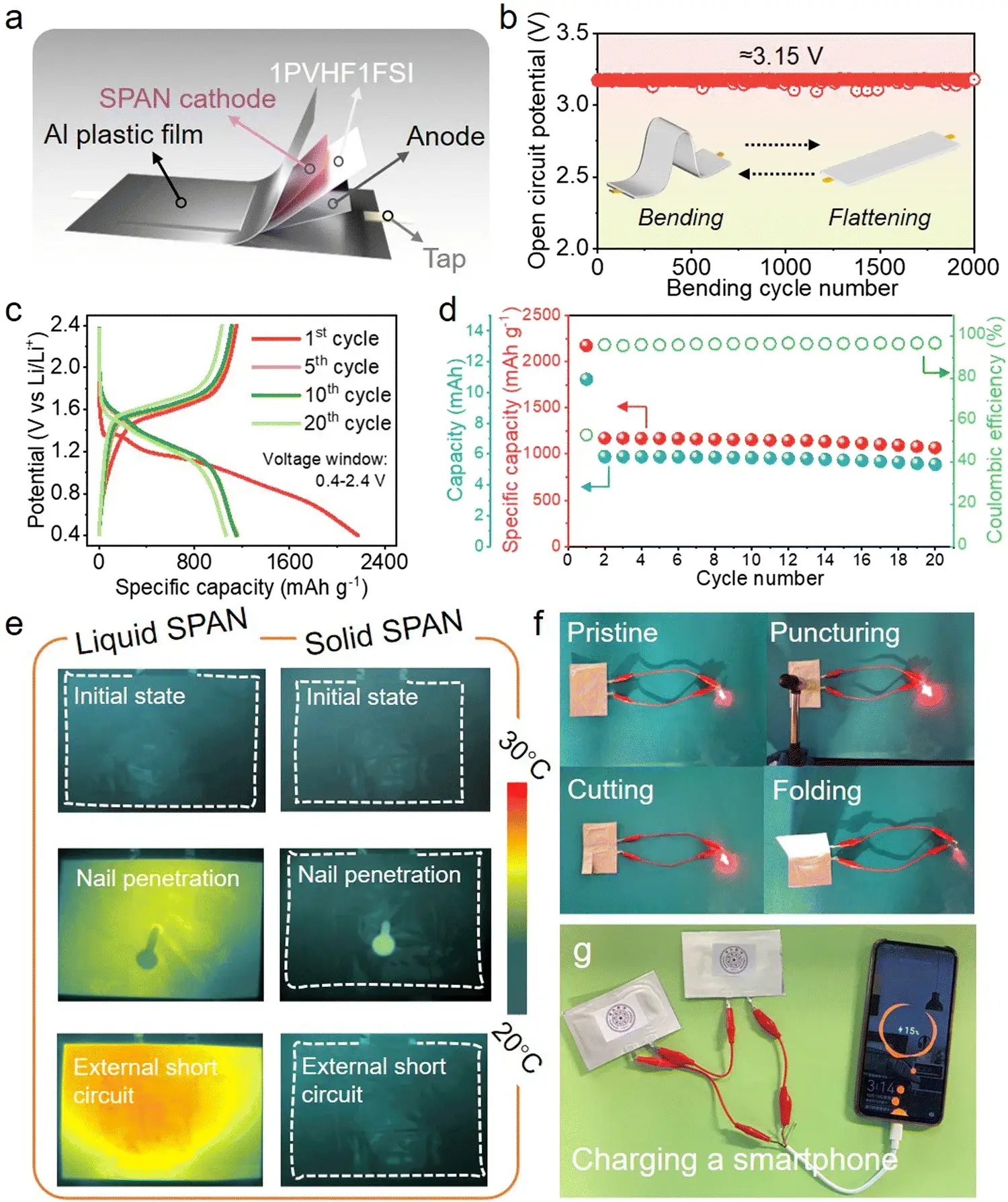
Figure 3. Performance characterization of solid SPAN pouch batteries
4.3 Practical Cell Performance and Durability
Building on the mechanistic understanding, solid-state and liquid-electrolyte SPAN pouch cells were assembled and tested for cycle life and flexibility. The solid-state SPAN batteries exhibited excellent mechanical flexibility, with capacity retention comparable to their coin-cell counterparts. Notably, the solid polymer electrolyte also demonstrated high thermal stability and remarkable tolerance to abusive conditions such as short-circuiting or nail penetration. The practical viability of the technology was further underscored by a demonstration where the solid-state pouch cell successfully charged a smartphone.
5. Performance Highlights — Stability, Flexibility, and Safety
The Li-SPAN cells demonstrated several practical advantages under the tested conditions:
-
Cycling stability: The solid-state SPAN cells retained capacity more stably than comparable liquid-electrolyte SPAN cells, attributed to limited Li₂S formation and improved reversibility of the quasi-intercalation process.
-
Rate capability: Faster redox kinetics translated to better high-rate performance than many conventional Li–S cells, as measured in the comparative rate tests.
-
Mechanical resilience: Soft-pack solid-state cells retained flexibility and showed robust electrochemical behavior under bending tests.
-
Thermal and safety behavior: The polymer electrolyte exhibited thermal stability and could withstand abuse conditions (short circuit and needle-puncture tests described in the source), indicating an advantageous safety profile for certain portable applications, for example smartphone charging demonstrations reported by the authors.
6. Summary
This study successfully demonstrates a high-performance solid-state Li-SPAN battery based on a salt-in-polymer electrolyte. By fixing sulfur within a PAN host, the formation of Li₂S is prevented, unlocking faster redox kinetics and mitigating volume expansion. The introduction of the “quasi-intercalation” mechanism provides a fresh and catalyst-free strategy for improving sulfur reaction kinetics, paving a new avenue for the development of advanced solid-state lithium sulfur battery.
7. Original Reference
8. IEST Recommend Instrument
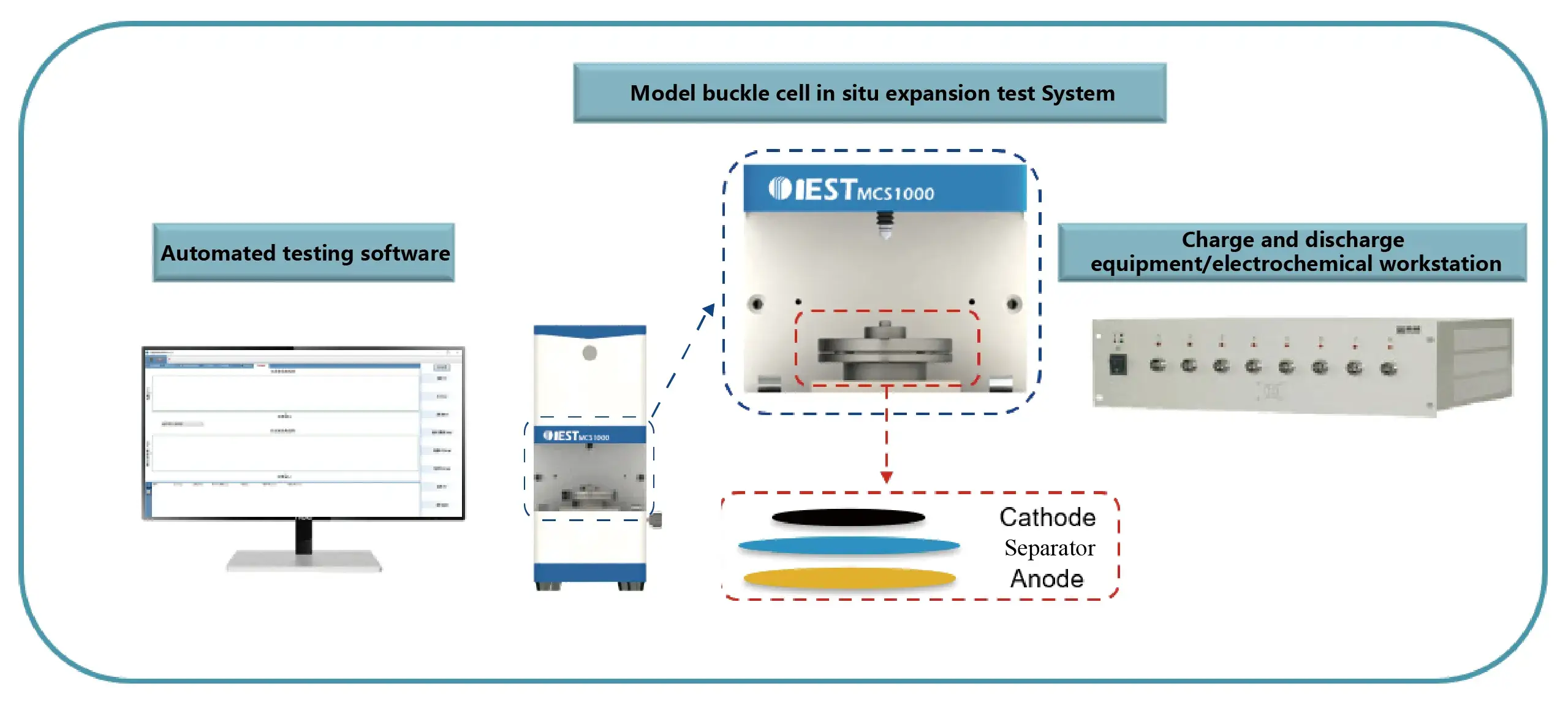
Model Coin-Cell Swelling Testing System(MCS1000)
Main features:
- The instrument size is small (length * width * height: 120 * 150 * 280mm), which can be placed in the glove box;
- The model coin cell can be used to assemble various types of full coin cell;
- Good tightness can ensure long-term test stability and obtain more reliable test results;
- High-precision thickness measurement system, thickness measurement resolution 0.1 µm precision ±1 µm.
- In-situ test of the full-cell expansion thickness curve;
- Ion conductivity of the solid electrolyte can be measured;
- The software can automatically combine the thickness change of the model coin cell with the charging and discharge data (compatible with partial charging and discharge tester), and output the report involving all testing data.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.