-
iestinstrument
An Apparatus For Studying In-situ Gas Production In Pouch Cells
1. Author Information and Article Abstract
CP Aiken (first author) and JR Dahn (corresponding author) of Dalhousie University in Canada introduced a device for in-situ gas production of lithium-ion pouch cells batteries to analyze the formation and initial charging of the ternary battery cell system. The phenomenon of gas production in the discharge process can distinguish the influence of different electrolyte additives on the volume change of the cell.
1.1 Measuring Principle
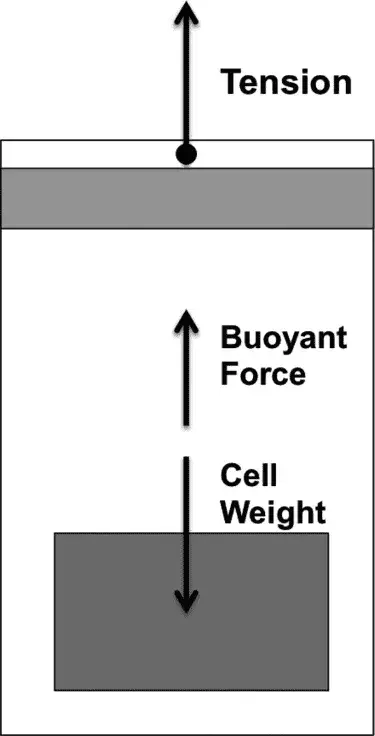
Figure 1. Schematic diagram of cell force
1.2 Archimedes’s Law of Buoyancy & Newton’s Theorem:

Where ρ is the density of the liquid, g is the acceleration due to gravity, V is the volume of the cell, Ftension is the tensile force, and the mass of the balance reading.
2. Measuring device
The battery cell is suspended in a silicone oil liquid while the pulling force of the battery cell is tested, and the positive and negative lugs of the battery cell are connected to a Maccor Series 4000 charging and discharging device to synchronize charging and discharging of the battery cell at an ambient temperature of 40.0 ± 0.1°C. The battery cell is then connected to a Maccor Series 4000 charging and discharging device to synchronize charging and discharging.
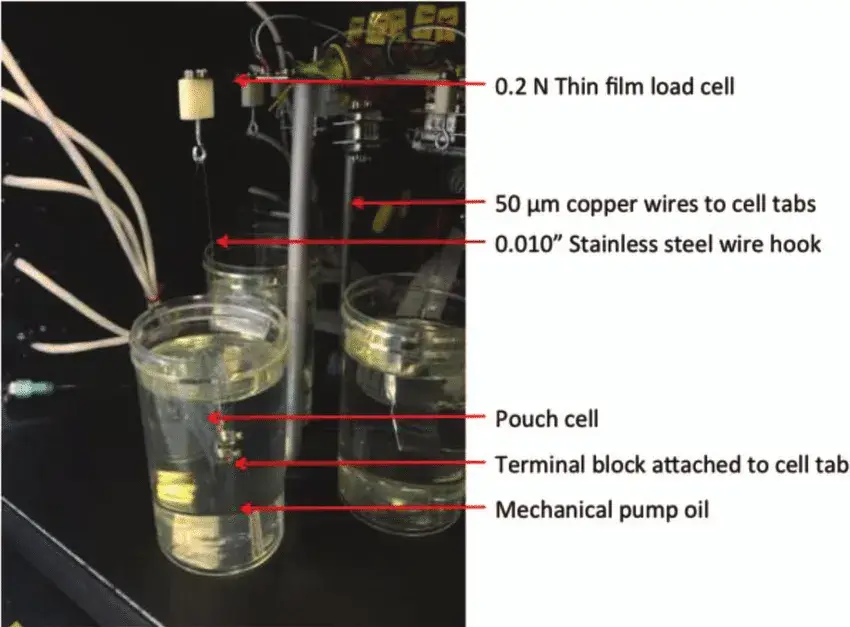
Figure 2. A photograph of the Archimedes In Situ Gas Analyzer (AISGA) with components labeled on one of the five testing channels.
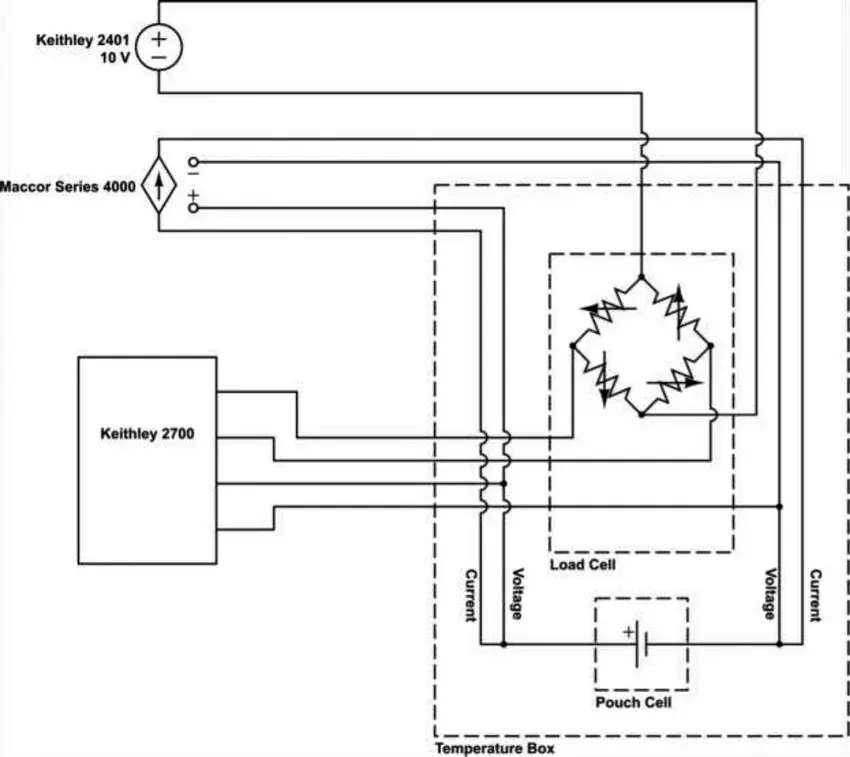
Figure 3. The physical diagram and circuit diagram of the in-situ measurement device
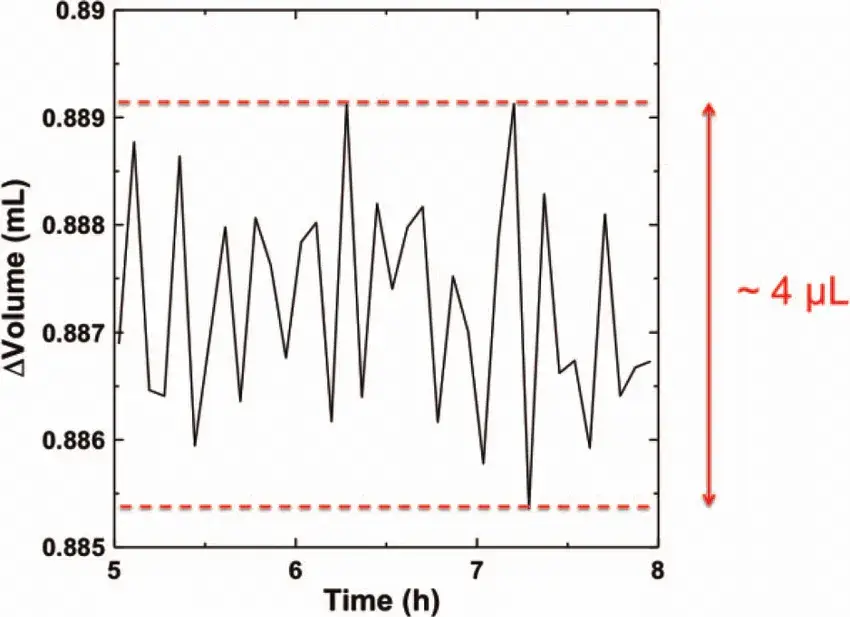
Figure 4. No-load noise of equipment
3. Result Analysis
3.1 The Influence of the Fixture
Whether there is a fixture when the cell is formed will affect the repeatability of the test and the trend of volume change. For batteries with clamps, the gas generated will be squeezed into the blank aluminum-plastic film bag on the side, and the volume of the batteries will not show an obvious downward trend, while the gas generated by the batteries without clamps will stay on the pole pieces. On the surface, further reactions occur, resulting in a decrease in the volume of the cell by about 0.1 mL. Therefore, the influence of fixtures needs to be taken into account during the experiment, and subsequent experiments are all without fixtures.
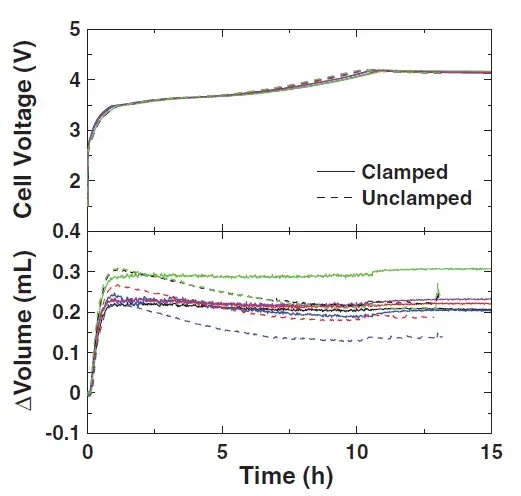
Figure 5. The effect of fixture on cell voltage and volume
3.2 The Influence of Magnification
Comparing the changes in cell volume under the four charging and discharging conditions for lithium preparation, it can be seen that during the first charging process, the cell volume reaches the maximum, and as the charge and discharge time increases, the overall cell volume shows a decreasing trend. This may be related to the fact that as the test time is extended, the first generated gas will react further and cause the volume to decrease. In addition, the “sawtooth” phenomenon can be seen during the charging and discharging process of the battery, which is mainly related to the expansion and contraction of the pole piece in the process of releasing lithium. Therefore, in order to see more charging and discharging signals, the charge and discharge rate of the core is preferably less than C/3.
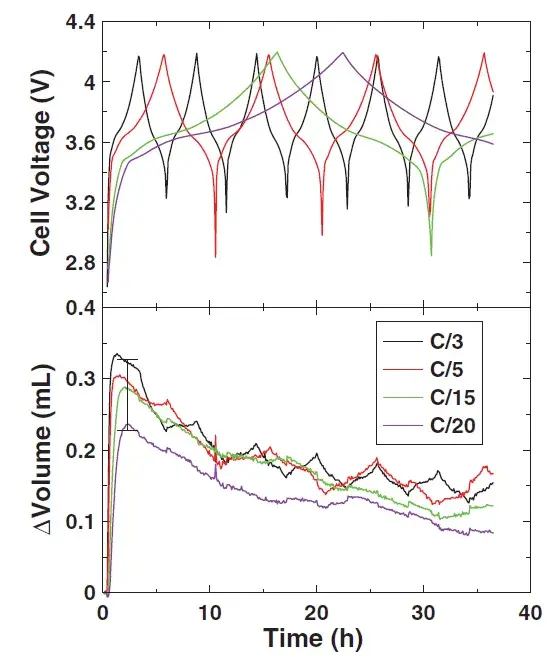
Figure 6. The influence of charge and discharge rate on cell voltage and volume
3.3 The Influence of Moisture and Additive Type
Whether the bare cell has been vacuum-dried for a long enough time before liquid injection is a key factor affecting the water content of the cell. Comparing the gas production difference between VC and VEC additives, it can be seen that when the cell contains moisture without vacuum drying, the gas production during formation will be greater than that of the dried cell.
Cells containing only VEC produce the largest amount of gas. Cells containing VC produce even less gas than batteries with blank electrolyte. When VEC and VC are added together, the cell’s gas production is less than that of blank cells. The above shows that VEC produces more gas when it reacts, but when VC is added again, it will inhibit the gas production reaction of VEC. The phenomenon of VC inhibiting gas production also occurs in the cell formation process when VC is added to ES and 11 other additives. By comparing the gas production volume curves of different types of additives, it can also be used to screen suitable electrolyte additives.
Table 1. The additives and their abbreviations as used in this study
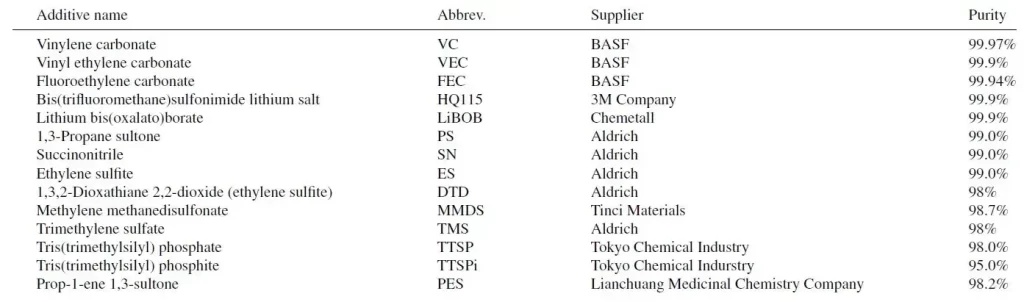
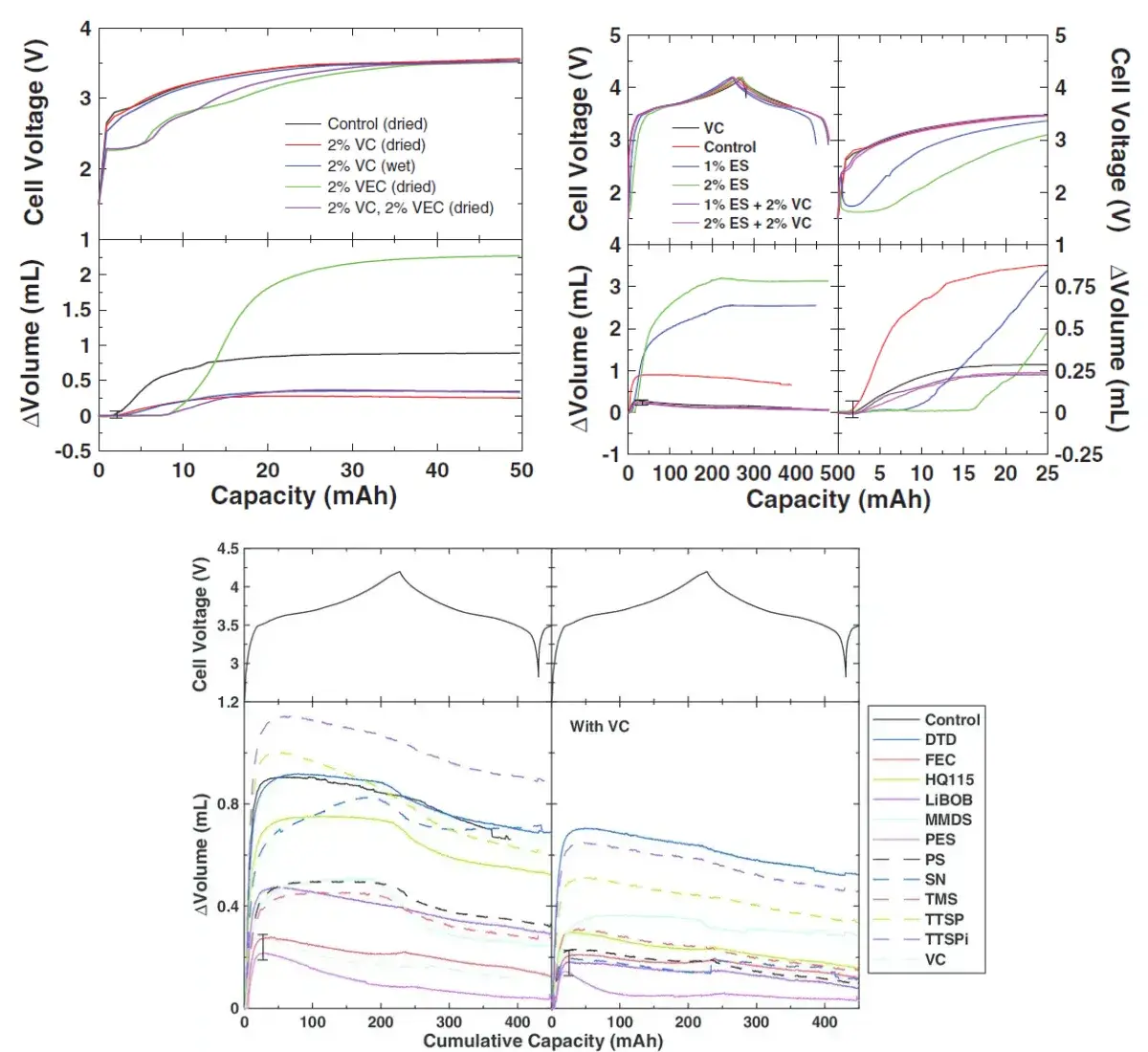
Figure 7. The effect of additive type on cell voltage and volume
4. Summary
The in-situ gas production monitoring device in this article can monitor the volume change of the pouch cell in the formation stage in real time, and then compare and analyze the gas production behavior of different additives:
- The formation current has no significant effect on the maximum total gas production of the cell within the measurable error range of the equipment;
- Most of the gas production of the battery cell occurs in the formation stage. As the charging and discharging time increases, the volume of the battery cell will gradually decrease, mainly because the subsequent reaction consumes a part of the gas;
- Among the 14 types of additives that have been tested, the combination of 2% VC + 2% PES is the best combination to reduce the gas production of formation, while VEC and ES will not produce gas during formation, and will delay the production gas start time of cells.
5. Reference
C. P. Aiken, J. R. Dahn et al. An Apparatus for the study of In situ gas evolution in Li-Ion pouch cells. J. Electro Soc, 161(2014) A1548-A1554.
6. IEST Related Test Equipment Recommendation
In-situ gas production volume monitor: model GVM2200 (IEST), test temperature range 20℃~85℃, support dual-channel (2 cells) synchronous test, resolution 1μL, long-term stability, and can be monitored simultaneously The changes in the volume of gas produced by the cell under the conditions of circulation, storage, overcharge and overdischarge, help the research and development of materials and cells!
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.




