-
iestinstrument
The Effect of Charge-discharge Rate On Lithium Cobalt Oxide(LCO)/Graphite Volume Expansion In Shelving Stage
1. Preface
Lithium-ion batteries are accompanied by volume expansion, including structural expansion and gas-producing expansion, during the processes of formation, cycling, storage, and overcharging. The charging and discharging rate determines the rate of de-embedded lithium reaction of the battery cell, which is also accompanied by different degrees of heat production or lithium precipitation. When researchers study the electrical performance of the battery cell, they usually add a certain amount of time of shelving at the end of the charging or discharging process to make the state of the battery cell stabilize, and to eliminate the thermal effect or polarization. How does the volume of the cell change during the resting phase?
In this paper, we investigate the effect of charging rate on the expansion by monitoring in-situ the volume expansion of pouch cell of the Lithium Cobalt Oxide(LCO)/Graphite system after shelving at different charging rate.
Figure 1. Various factors affecting the fast charging of lithium-ion batteries[1]
2. Test information
2.1 Test Equipment
In-situ Battery Gassing Volume Analyzer, model GVM2200, can control the temperature of 20℃ ~80℃, as shown in Figure 2.
Figure 2. In-Situ Battery Gassing Volume Analyzer (GVM2200)
2.2 Test Parameter
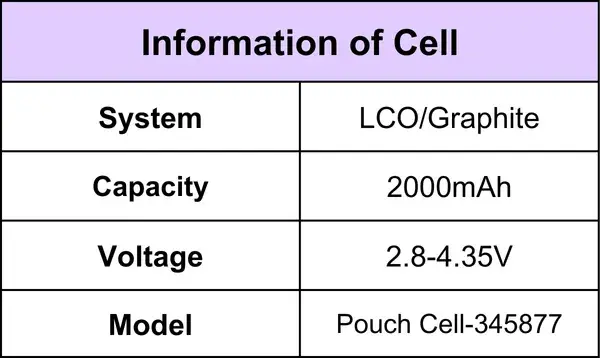
The Cell Information is Shown in Table 1
Table 1. Cell Information
2.3 Experimental Parameters
Four experimental groups are set according to the parameters in Table 2 below, and shelved for 1 hour after each charging and discharge. The battery is then placed in the in-situ gas production volume monitor(GVM2200), adjust the oil bath temperature to 25℃, and monitor the volume change of the cell in real time.
Table 2. Charge and Discharge Parameters

3. Interpretation of Result
3.1 Monitor In-situ the Whole-process Cell Volume Swelling Curve
The charge-discharge curves and the volume change curves for the four cycles are shown in Figure 3. Constant current charging stage: As the SOC increases continuously, the volume expansion continuously, which is mainly related to the structural expansion of lithium-ion constantly embedded in graphite. After entering the constant pressure and shelved stage, the volume of the cell began to shrink and gradually reached stability. In the constant current discharge stage: with the gradual increase of DOD, the volume of the cell cell continues to shrink, and when the doubling rate of the discharge gradually becomes small, the abnormal swelling of the initial volume of the cell discharge is also gradually decreasing. Entering the shelved stage after discharge, the volume of the cell continues to decrease and stabilize with time. Next, we perform a detailed analysis of the shelving stages A and B after charging and discharge.
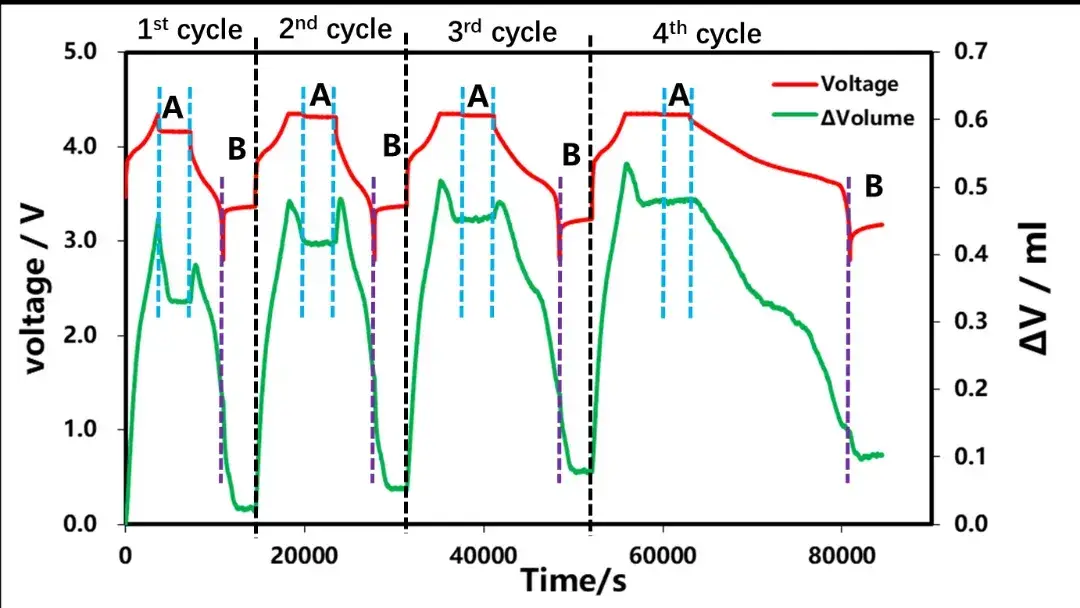
Figure 3. Different charging and discharging currents and volume change curves of the battery cell
3.2 Volume Swelling Analysis in the Charging Shelved Stage
The rechargeable shelved part A of each lap in Figure 3 was selected for analysis, as shown in Figure 4. As the cut-off current of each lap is gradually reduced, according to the shelved volume curve, when there is no CV in the first lap, the cut-off current of charging is 1C. At this time, the volume gradually decreases with the shelving time and remains unchanged after about 1500s. In the last three circles, the constant pressure stage is increased. When the charging cut-off current is less than 0.1C, the volume contraction appears at the beginning of the constant pressure, and then when the shelved after charging is increased, the volume is basically unchanged. This shows that volume shrinkage and current reduction, when there is constant pressure, current reduction accompanied by volume shrinkage, this is likely to be constant voltage stage battery internal polarization reduction, lithium concentration difference in electrode thickness direction, lithium concentration difference stress and strain reduction if no constant pressure stage, at the end of charging, the battery internal polarization, lithium concentration difference is also very big, shelved stress and strain release, at least half an hour to achieve stable state.
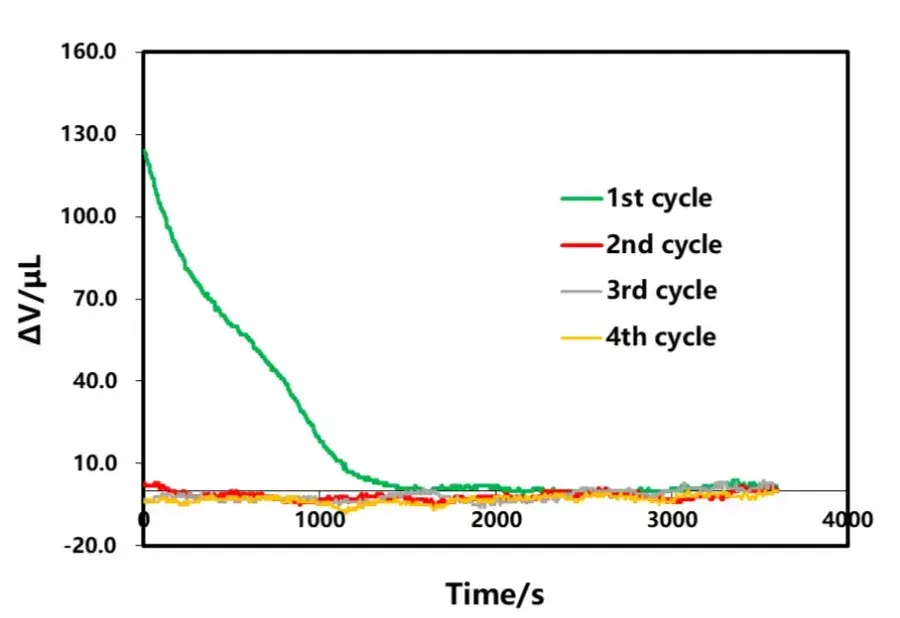
Figure 4. Effect of the charge cut-off current on the shelved volume
In order to further explain the process of lithium-ion transmission and charge state evolution in the constant current-constant pressure charge process, the mechanical-electrochemical model is used to study the distribution of lithium concentration, stress and strain in the charging process.
3.3 Constant-current Charging Stage
During the constant flow charging phase, lithium-ion are continuously embedded in the graphite layer, which leads to structural swelling. As the SOC continuously grows, the volume constantly expands. When the constant current charging is completed, the lithium ion concentration in the electrolyte, the lithium concentration in the negative electrode particles, the strain, and the stress are as shown in Figure 5. Figure 5 (a) shows that the closer the cathode fluid collection is, the higher the lithium-ion concentration in the electrolyte is. This is because the positive / negative particles near the diaphragm are preferentially lithium / lithium. At the end of constant current charging, the degree of lithium removal / lithium removal of the particles near the diaphragm is greater than that of the particles near the fluid collection. From Figure 5 (b), higher lithium concentration in the negative particles close to the diaphragm. This is because the electrolyte in the negative region away from the diaphragm has a longer lithium diffusion distance than the negative particles near the diaphragm, allowing the lithium-ion reaching the anode to be preferentially inserted into the particles close to the diaphragm.
In the negative electrode particles near the diaphragm, the solid-phase lithium concentration is higher, so their strain is also higher, as shown in Figure 5 (c). Meanwhile, the strain is greater on the free surface of the particles, but less so on the contact surface between the particles and between the particles and the boundary. This is because the insertion of lithium ions causes the volume swelling of the particles. The free surface of the particles expands outward, leading in greater deformation. However, the deformation on the contact surface is smaller under the constraints of adjacent particles and boundaries. Figure 5 (d) shows that the stress distribution is opposite to the strain distribution. This is because the free surface of the particles is unconstrained and therefore stress less. However, the contact surface is strongly constrained and therefore greater stress. The maximum stress is located in the particles close to the diaphragm. The average strain of the negative electrode particle (meaning the mean value of the whole body of the particle in the electrode) during the constant current charging is shown in Figure 6. Both increase gradually with time, and their change trend is basically consistent with the relationship shown in Figure 3. After CC charging, the average strain and average stress reach the maximum value respectively.
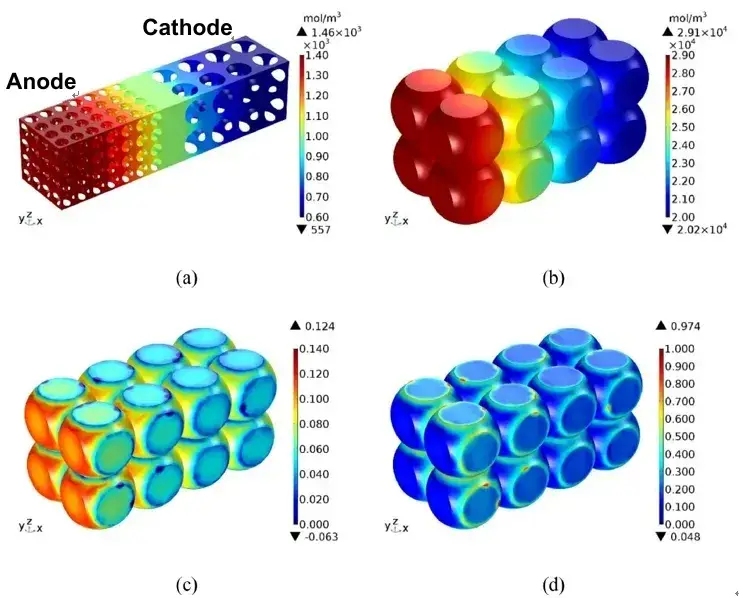
Figure 5. Lithium-ion concentration (a) and graphite particle solid phase lithium concentration (b), strain (c) and stress (d) distribution of graphite particles at the end of 1C CC charging[2]
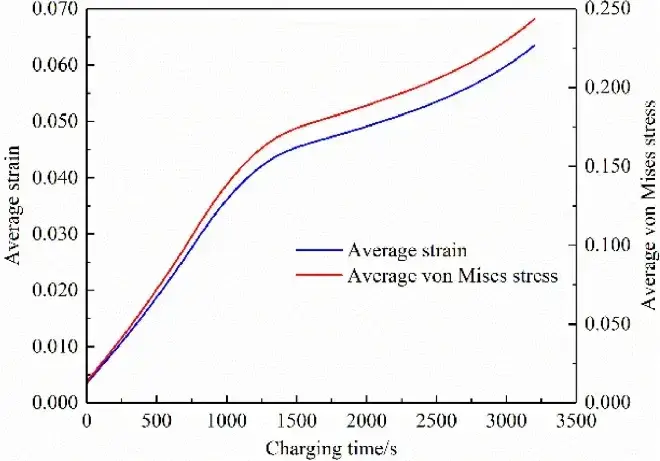
Figure 6. Average strain and average stress of the anode particles during charging[2]
3.4 Volume expansion analysis of discharge and shelving stages
The shelving B part after discharge of each lap in Figure 3 is selected for analysis, as shown in Figure 9. From the volumetric curve of shelving, the amount of volumetric change in shelving decreases as the discharge rate decreases, and the time for volumetric stabilization is gradually shortened. The phenomenon of volume contraction in the shelving stage after discharge is similar to that in the shelving stage after charging, and both are related to the polarization and uneven distribution of lithium concentration inside the cell. Analyzing the volume change curves corresponding to different rate discharge, as shown in Figure 10, in the early stage of discharge, the volume of the electric core all appeared to be anomalous expansion phenomenon, and with the decrease of the discharge rate, the expansion amount is also gradually reduced, which is likely to have a relationship with the internal thermal effect of the cell caused by the large rate as well.
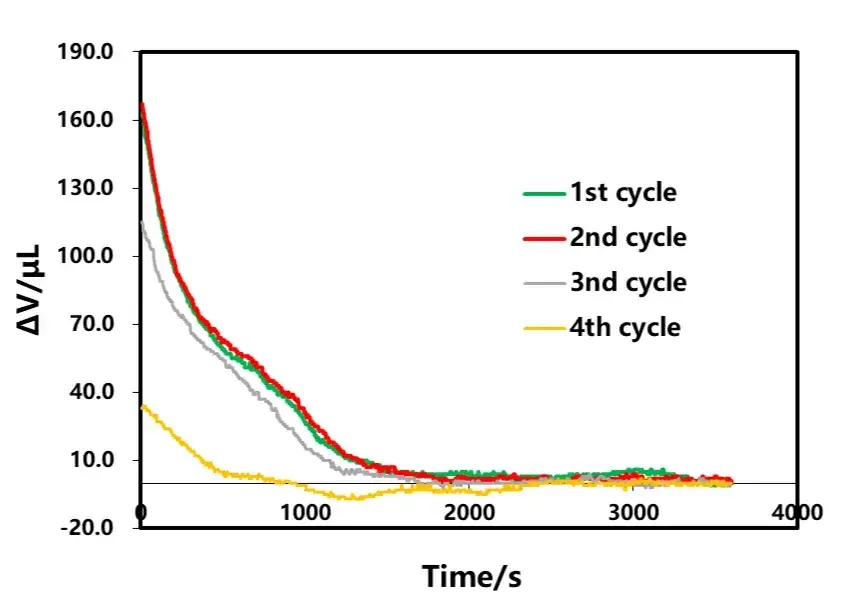
Figure 9. Discharge current effect on shelving volume
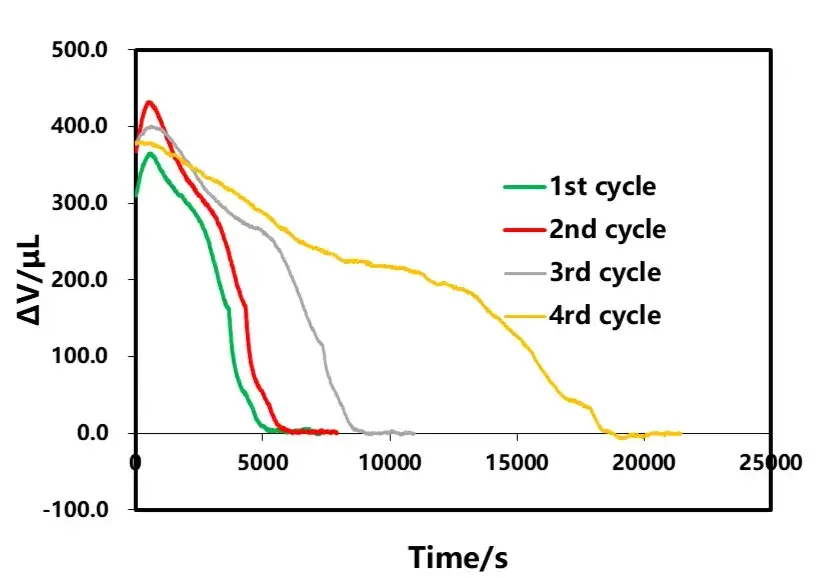
Figure 10. Discharge current effect on total volume and bounce volume
According to the similar results of the simulation of the constant current and constant voltage charging process, the lithium is de-embedded from the negative electrode particles but embedded in the positive electrode particles as the discharging process continues during the constant current discharging process. At the same time, the electrochemical potential drives the lithium in the electrolyte to move from the negative electrode to the positive electrode. The lithium concentration in the negative electrode particles decreases, but increases in the positive electrode particles. In the electrode thickness direction, there is the same problem of uneven distribution of lithium concentration, and this lithium concentration difference is related to the thickness and rate of the electrode. As shown in Figure 11, at the end of discharge, when the electrode thickness is relatively small or the rate is relatively small, the lithium concentration distribution is more uniform. And when the electrode thickness or rate increases, there is an obvious concentration difference. For the positive electrode, the concentration of particulate lithium near the diaphragm is high, and the concentration of particulate lithium near the collector is low. Therefore, as the discharge rate decreases, the lithium concentration difference inside the battery becomes smaller, and the stress and strain caused by the concentration difference are smaller. The amount of volume change during shelving also gradually decreases, and the time for volume stabilization is gradually shortened.
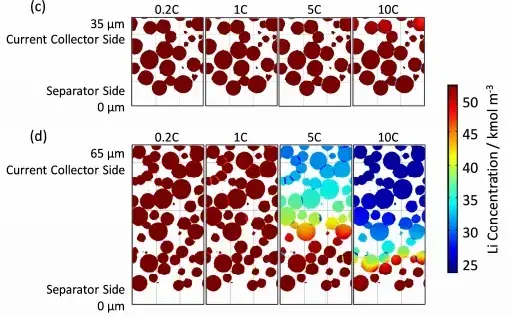
Figure 11. Distribution of lithium concentration in the solid phase of the particles at the end of discharge[3]
4. Summary
This paper adopts the IEST in-situ gas volume monitoring instrument (GVM2200), monitoring the Lithium Cobalt Oxide(LCO)/Graphite system cell volume expansion behavior at different rates, found that the process of volume expansion behavior is not only related to the embedded lithium behavior, but also related to the charge and discharge lithium concentration distribution, may also be related to the cell thermal effect caused by current. Therefore, setting the appropriate charging cut-off current can effectively eliminate the impact of the uneven distribution of lithium concentration inside the cell on the volume expansion. However, due to the discharge process current is generally large, the corresponding increase of shelved time after the discharge is needed to make the cell reach a stable state.
5. References
[1] Anna Tomaszewska, Zhengyu Chu, Xuning Feng, et al.Lithium-ion battery fast charging: A review,eTransportation, 1 (2019) 100011.
[2] Factors affecting stress in anode particles during charging process of lithium ion battery, Journal of Energy Storage, 43(2021)103214.
[3] Hideki Kikukawa, Kohei Honkura, Michihisa Koyama.Influence of inter-particle resistance between active materials on the discharge characteristics of the positive electrode of lithium ion batteries, Electrochimica Acta, 278(2018)385-395.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



