-
iestinstrument
Research On The Modification Of High-performance Lithium-rich Manganese-based Cathode Materials

First Author: Fang Youyou
Corresponding Author: Su Yuefeng, Dong Jinyang, Chen Lai
Publishing Unit: Beijing Institute of Technology, Beijing Institute of Technology Innovation Center
Equipment Used: IEST Powder Resistivity & Compaction Density Measurement System (PRCD1100)
1. Research Background
With the rapid development of electric vehicles and portable energy storage systems, there is an urgent need to improve the energy density and cost-effectiveness of lithium-ion batteries, and lithium-rich manganese-based oxide (LLO) materials stand out in these fields. Despite the advantages of high specific energy and low cost of this material, they have significant obstacles in practical applications, including low initial Coulombic efficiency, poor cycling/multiplication performance, and voltage degradation, which impede their wide application. To this end, we introduce an ionic-electronic dual-conducting (IEDC) surface control strategy that integrates a graphene framework with high electronic conductivity with a heterogeneous epitaxial spinel Li4Mn5O12 layer with high ionic conductivity. Electrochemical tests and structural analysis show that this IEDC heterostructure effectively reduces polarization, mitigates structural distortion, and enhances electron/ion diffusion. Density-functional theory calculations emphasize the abundant Li+ permeation network and low Li+ migration energy at the layered spinel interface.
2. Article Introduction
Recently, Wu Feng’s team from Beijing Institute of Technology published a paper entitled “Ionic-electronic dual-conductor interface engineering and architecture design in layered lithium-rich manganese-based oxides”. manganese-based oxides”. In this study, the electrochemical performance of lithium-rich manganese-based cathode materials (LMOs) was significantly improved by constructing an ionic-electronic dual-conductor (IEDC) interface combining a graphene framework with high electronic conductivity and a heterogeneous epitaxial structure of spinel Li4Mn5O12 with high ionic conductivity.
3. Content Representation
Figure 1 shows a schematic of the preparation process and design principles of IEDC interface engineering. The process involves a post-thermal treatment and chemical cladding process in a graphene (Gr) lattice, which results in the growth of self-induced spinel heterogeneous phases on the surface of the laminate structure of the native sample. As a result, the LMO particles are encapsulated by the spinel-Gr hybrid layer to form the LMOSG sample. The Li+ diffusion kinetics and surface structural stability were enhanced due to the high ion diffusion properties of the epitaxial spinel heterogeneous phase. Meanwhile, the excellent conductivity of the graphene network reduces the delithiation polarization and strengthens the electrode/electrolyte interface. This innovative surface reconstruction design significantly improves the cycling and multiplicity performance and provides a promising strategy to enhance the stability of lithium-rich manganese-based cathode.
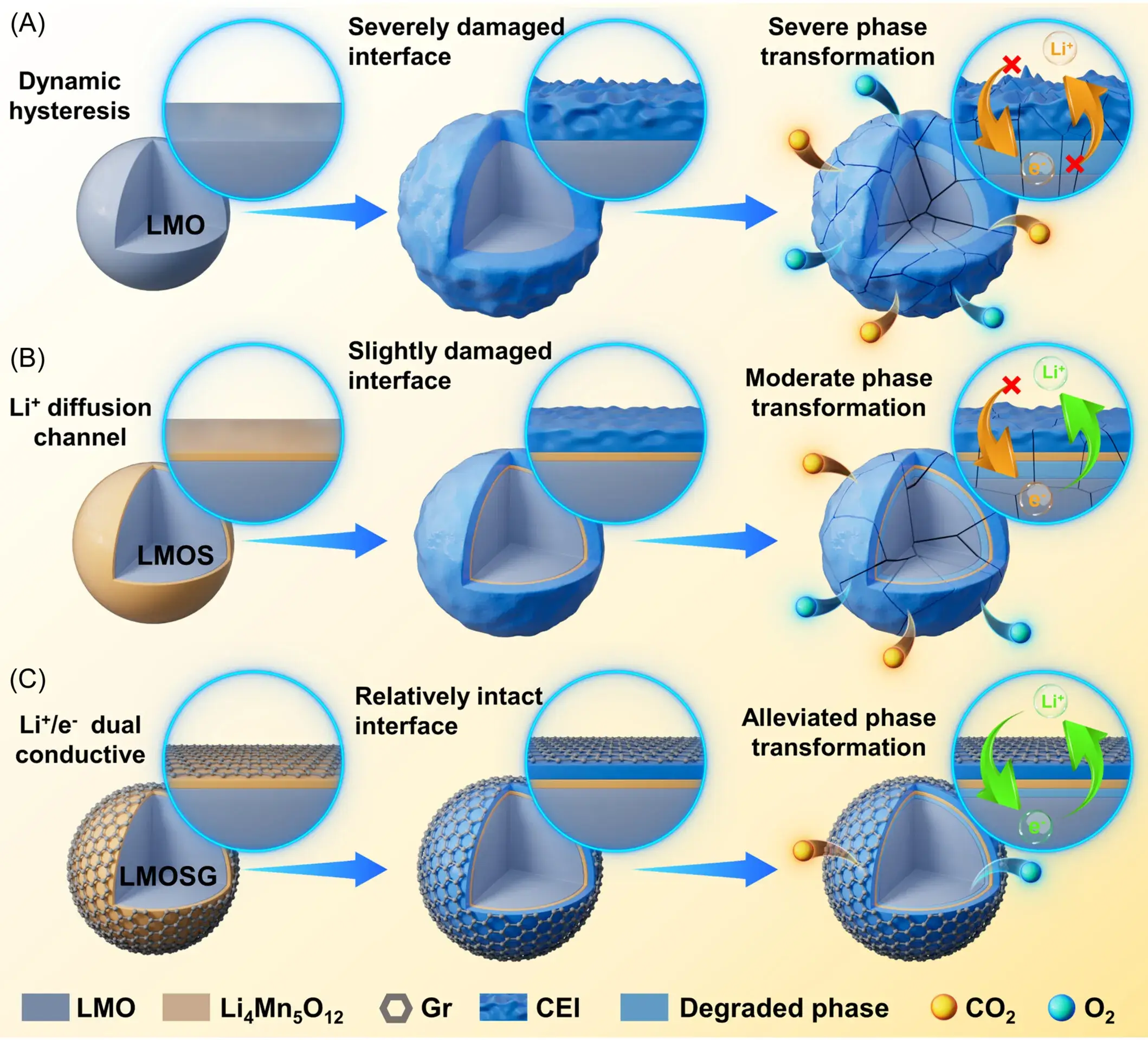
Figure 1. Schematic evolution of CEI for (a) LMO, (b) LMOS and (c) LMOSG and electron/Li+ conductivity correspondence during electrochemical processes
4. Highlights 1 of This Paper
The effectiveness of IEDC interfacial engineering in promoting Li+ diffusion and stabilizing lattice oxygen is confirmed by mutual validation of density-functional theory (DFT) calculations and experimental results.
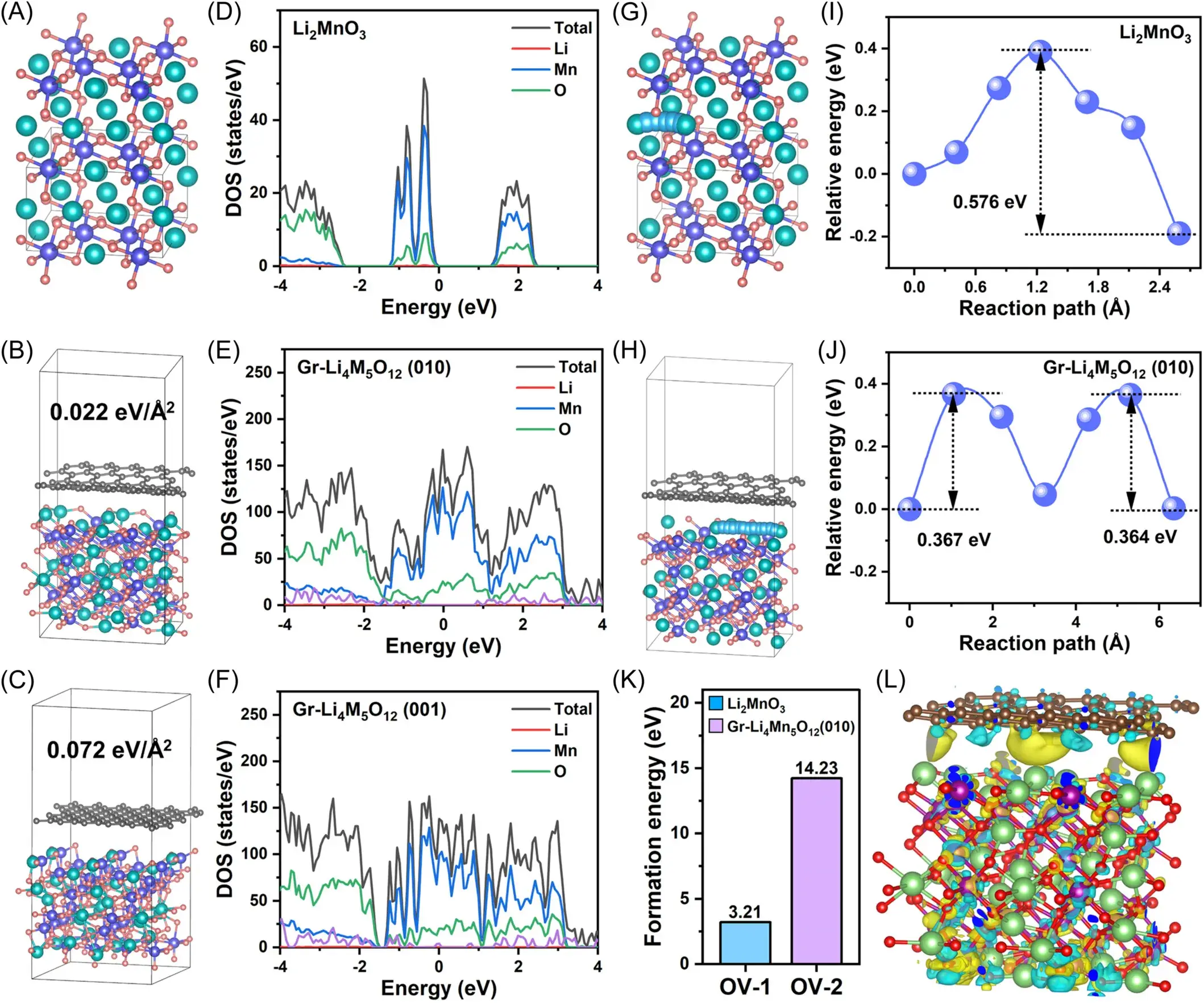
Figure 2. (a)The structure models of (A) Li2MnO3, (B) Gr-Li4Mn5O12(010), and (C) Gr-Li4Mn5O12(001) and the corresponding total density of states plots are illustrated in (D–F). The Li+ diffusion pathways and corresponding diffusion energy barriers of (G, I) Li2MnO3 and (H, J) Gr-Li4Mn5O12(010). (K) The oxygen vacancy formation energy of Li2MnO3 and Gr-Li4Mn5O12(010). (L) The electron density difference for the Gr-Li4Mn5O12(010) surface.
Figure 2 reveals the effect of IEDC interfacial engineering on Li+ diffusion kinetics and material stability through density functional theory (DFT) calculations and structural modeling. The constitutive models of Li2MnO3, Gr-Li4Mn5O12 (010) and (001) crystal planes and their total density of states maps are presented in the figure. The density of states at the Fermi energy level increases significantly after the introduction of Gr and Li4Mn5O12, indicating that the Gr component enhances the electronic conductivity of Li2MnO3.The analysis of the Li+ diffusion paths and corresponding diffusion barriers shows that the Gr Li+ diffusion energy barrier of Li4Mn5O12 (010) decreases from 0.576 eV to 0.367 eV compared to Li2MnO3, attributed to the three-dimensional lithium diffusion channel provided by Li4Mn5O12. The analysis of oxygen vacancy formation energy shows that Gr-Li4Mn5O12 (010) has a significantly higher oxygen vacancy formation energy than Li2MnO3, indicating that IEDC interfacial engineering enhances the lattice oxygen stability. Differential charge density maps reveal the electron distribution at the interface between Gr and Li4Mn5O12 (010), suggesting that electrons are more easily transferred from graphene to the outer oxygen atoms of Li4Mn5O12. These findings emphasize the effectiveness of IEDC interfacial engineering in promoting Li+ diffusion and stabilizing lattice oxygen, thereby enhancing the electrochemical performance of lithium-rich manganese-based cathode materials.
5. Highlights 2 of This Paper
For the first time, spinel Li4Mn5O12 was combined with graphene to form a novel ion-electron double conductor (IEDC) interface.
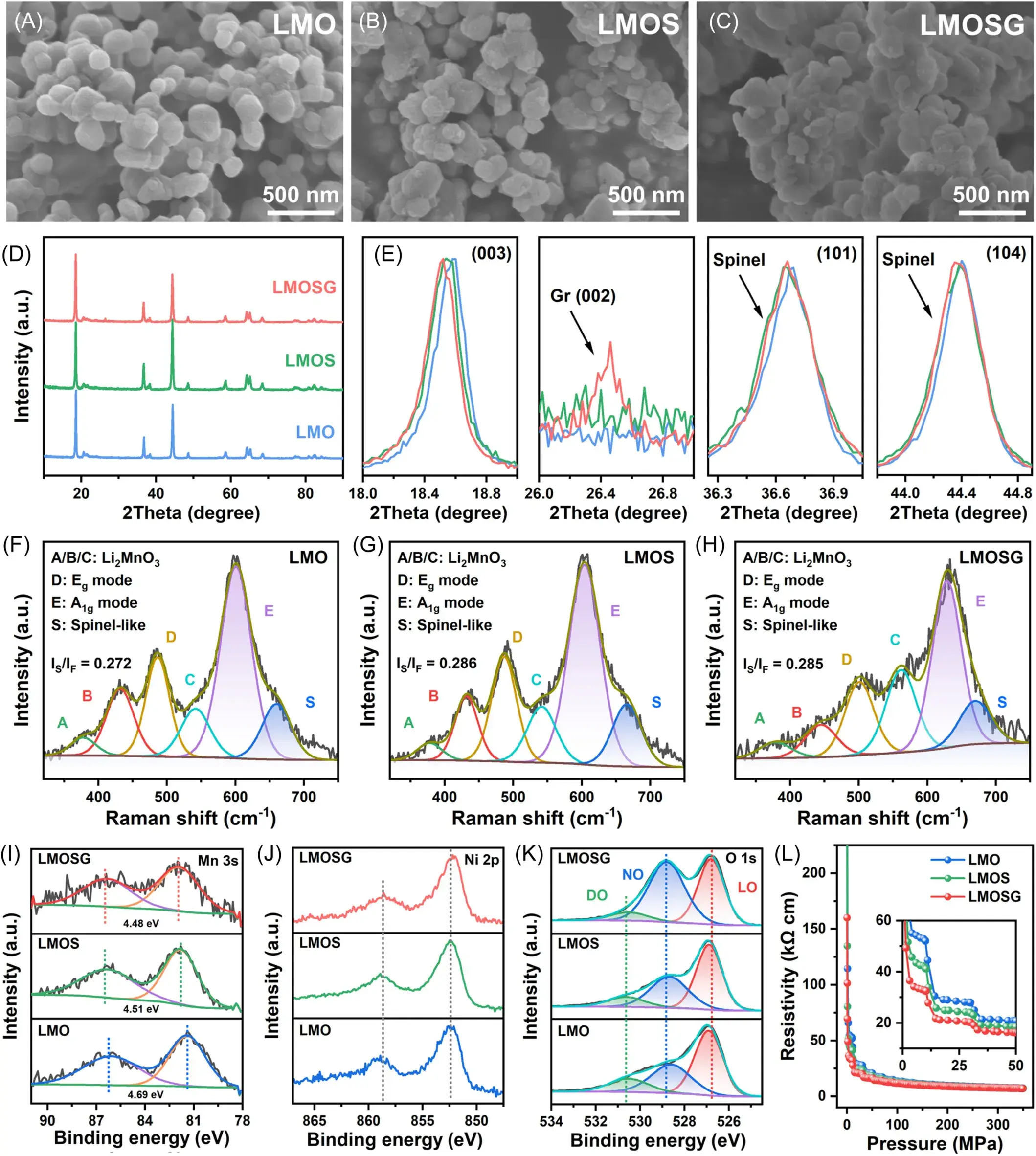
Figure 3. SEM images of the (A) LMO, (B) LMOS, and (C) LMOSG samples. (D) Panoramic XRD patterns with (E) expanded 2θ regions. Raman spectra of the (F) LMO, (G) LMOS, and (H) LMOSG samples. (I) Mn 3s XPS spectra, (J) Ni 2p XPS spectra, (K) O 1s XPS spectra, and (L) pressure versus resistivity plot for all three samples.
Figure 3 provides the morphological and structural characterization of the LMO (Li1.2Mn0.6Ni0.2O2), LMOS (spinel Li4Mn5O12 cladding sample) and LMOSG (spinel/graphene bilayer cladding sample) samples. Through SEM images (Figure 3a-c), we observed that the particle sizes of all three samples were between 200 and 300 nm, and the particle morphology did not change significantly after the IEDC interfacial engineering treatment.XRD analysis (Figure 3d) showed that the major diffraction peaks of all the samples corresponded to the characteristic peaks of the lithium-rich manganese-based oxides. The additional weak diffraction peaks in the LMOSG samples were identified as the characteristic peaks of graphene alkene characteristic peaks, while the presence of the Li4Mn5O12 phase was confirmed by the raised shoulder peaks in the XRD patterns. Raman spectra (Figure 3f-h) further revealed the local structure of the samples, where the LMOSG samples showed an increased spinel content due to the introduction of graphene components. XPS spectra (Figure 3i-k) analyzed the chemical state of the sample surfaces, and the changes in the Mn 3s peaks indicated an elevated average oxidation state of Mn in the LMOSG samples, confirming the formation of the spinel phase. Resistivity-pressure plots (Figure 3l) showed that LMOSG had the lowest resistivity at different pressures, indicating that the IEDC interfacial engineering significantly increased the conductivity. Together, these test results confirm the successful construction of the IEDC interface and its favorable impact on enhancing the electrochemical properties of the material.
6. Highlights 3 of This Paper
The electrochemical performance of lithium-rich manganese-based cathode materials, including initial coulombic efficiency, discharge specific capacity, and cycling stability, was significantly improved.

Figure 4. (A) Initial charge‒discharge profiles at 0.1 C between 2.0 and 4.8 V. (B) Rate performance between 2.0 and 4.6 V. (C) Cycling performance at 1 C between 2.0 and 4.6 V. (D) Cycling performance at 5 C between 2.0 and 4.6 V. Corresponding charge‒discharge curves at 1 C for the (E) LMO electrode, (F) LMOS electrode, and (G) LMOSG electrode. Corresponding discharged dQ/dV plots of the (H) LMO electrode, (I) LMOS electrode, and (J) LMOSG electrode.
Figure 4 synthesizes the electrochemical performances of the LMO, LMOS and LMOSG anodes, including the first charge/discharge curves, the multiplicity performance and the cycling stability. The first charge-discharge curve (Figure 4a) shows that the LMOSG anode exhibits a high discharge specific capacity of 296.7 mAh g-1 at 0.1 C multiplicity and a high initial coulombic efficiency of 82.9%, which is superior to that of the LMO and LMOS anodes. The multiplicity performance test (Figure 4) shows that the LMOSG anode exhibits high discharge capacity at different current densities from 0.1 C to 5 C, especially at the high multiplicity of 5 C. It still maintains a reversible capacity of 176.5 mAh g-1, while that of the LMO anode is only 151.8 mAh g-1. The cycling performance test (Figure 4c and d) further confirms the superiority of the LMOSG anode The LMOSG anode exhibits better capacity retention and lower voltage degradation under both 1 C and 5 C test conditions. These results indicate that IEDC interfacial engineering significantly enhances the electrochemical performance of the cathode materials, including improved specific capacity, coulombic efficiency, multiplicative performance, and cycling stability, which is mainly attributed to the synergistic effect of the heterostructures of graphene and spinel Li4Mn5O12, which work together to optimize the electron/ion transport paths, reduce the interfacial impedance, and improve the structural stability.
7. Summary and Outlook
In this study, a simple IEDC interfacial engineering technique was applied to lithium-rich manganese-based cathode materials(LMO) particles to create a multilayer interface consisting of a conductive Gr framework and a spontaneously formed heterogeneous epitaxial layer of ion-conducting spinel Li4Mn5O12 on the surface of LMO particles. This dual-conducting surface was verified by comprehensive tests to have the ability to reduce polarization, maintain crystal structure stability, and promote rapid electron/ion diffusion.DFT calculations showed that the electronic conductivity of the Gr/ Li4Mn5O12 (010) interface was improved compared to the pristine lithium-rich manganese-based cathode materials due to the significant reduction of the electronic band gap. Further theoretical studies revealed a highly interconnected 3D Li+ permeation network and reduced Li+ migration energy in the heterostructure of the layered spinel interface. Crystal structure tests and electrochemical analyses show that the innovative multilayer structure limits lattice oxygen release and TM migration/dissolution, while enhancing ionic conductivity, resulting in improved electrochemical performance. This effective and detailed surface reconstruction method is expected to contribute to the development of multifunctional interfaces for next-generation electrochemical energy storage systems.
8. Original Paper
Youyou Fang, Yuefeng Su, Jinyang Dong, Jiayu Zhao, Haoyu Wang, Ning Li, Yun Lu, Yujia Wu, Wenbo Li, Ni Yang, Xiaojuan Wu, Feng Wu, Lai Chen. Ionic‐electronic dual‐conductor interface engineering and architecture design in layered lithium‐rich manganese‐based oxides. Carbon Energy, 2024, e642.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


