-
iestinstrument
Entering Electrochemistry | The Significance of High-Precision Charge/Discharge Testing for Predicting the Lithium ion Battery Lifespan
1. Background
As lithium batteries are gradually being applied in various aspects of life, the demands placed on them by people are increasing, such as longer lithium ion battery lifespan and higher energy density. To meet these demands, on the one hand, continuous research and development of new materials and improvement of new processes are needed to enhance the specific capacity of materials. On the other hand, the capacity of batteries or battery packs is also increasing to meet the long-range requirements of new energy vehicles or energy storage stations.
We know that charging and discharging batteries at different currents or rates are the most basic operations for testing battery performance, and most of the side reactions can also be inferred from these charge and discharge curves. However, to obtain refined analysis results, the prerequisite is the use of “high-precision” current and voltage testing equipment to discern minute side reactions.
By “high precision” we generally mean a test accuracy of 1 part in 10,000 or more. As for the common test equipment on the market with accuracy of 1/1000 or 5/10,000, the fluctuation of the current and voltage test is relatively large, which is very easy to cover up the improvement effect of the new material’s gram capacity. In addition, the large test fluctuations can not effectively screen out the tiny side reactions in the pre-cycle period of the battery, and the prediction of the long cycle lithium ion battery lifespan is naturally inaccurate. Therefore, with the increasing demand for lithium battery performance, the demand for high-precision charge/discharge test equipment is bound to increase in the future.
The next step will focus on introducing the significance of high-precision charge/discharge testing for predicting the lithium ion battery lifespan from the following three aspects.
2. Precise Coulombic Efficiency (CE) Testing – Rapid Lithium ion Battery Lifespan Prediction
Figure 1(a) shows the typical charge/discharge curves, where the Coulombic Efficiency (CE) can be calculated by dividing the discharge capacity by the charge capacity for each cycle, CE = QD(n)/QC(n). In the ideal scenario without any side reactions, CE equals 1, indicating fully reversible charging and discharging, resulting in infinite lithium ion battery lifespan. However, various side reactions often consume active lithium within the battery, leading to irreversible capacity loss, i.e., CE < 1. It is evident that the extent of side reactions directly determines the lithium ion battery lifespan. Therefore, with the aid of high-precision testing equipment, we can accurately assess the magnitude of CE, thus enabling rapid prediction of the lithium ion battery lifespan. Figure 2 illustrates a battery life prediction model [1] established using CE data from a certain number of cycles, where the initial capacity of a fresh battery is C0, the Coulombic Efficiency for the k-th cycle is CEk and considering lithium loss as the cumulative amount per cycle, the capacity of the battery after cycling aging is Ck = C0 * (CE1 * CE2 * … * CEk).
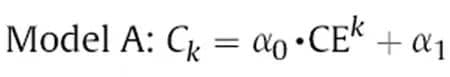
Among them, α0 and α1 are empirical parameters of the model, which can be obtained by fitting a certain number of cycles of Coulomb efficiency. Suppose α0 is the initial capacity of the battery, and α0 = C0 = 100Ah, while α1 = 0. If the average Coulomb efficiency accuracy is low, with a value of 99.95%, when the number of cycles k = 500, calculate Ck = 100Ah * 0.9995500 = 77.88Ah; if the average Coulomb efficiency accuracy is high, with a value of 99.955%, then Ck = 100Ah * 0.99955 500 = 79.85Ah. The difference between the two is 1.97Ah, which is a 1.97% difference in model accuracy. Therefore, the accuracy of Coulomb efficiency is crucial.
In battery performance comparative analysis, high-precision Coulomb efficiency can also provide more information. For example, Figures 1(c) and (d) respectively illustrate the comparison of long-cycle capacity and CE comparison results in the early cycles of batteries prepared using three different electrolytes [2]. From Figure 1(c), it can be observed that the electrolyte combination (VC+VEC+FEC+PS) effectively extends the lithium ion battery lifespan to around 500 cycles, while the other two electrolytes only cycled around 150 cycles and 300 cycles respectively.
What’s interesting is that if we observe the CE comparison results in Figure 1(d), the CE of the three differs slightly in the early cycles (around 16 cycles). The electrolyte (VC+VEC+FEC+PS) maintains a CE above 0.999, while the other two electrolytes have CE values of only around 0.998 and 0.9965. This means that if the testing accuracy of battery CE is sufficiently high, we can distinguish the extent of side reactions early in the cycling process, enabling a rapid prediction of the lithium ion battery lifespan. Compared to the traditional method of mechanically cycling batteries round by round to determine their lifespan, this approach will greatly reduce experimental time and enhance battery development efficiency!
From the comparison results in Figure 1(d), the difference in CE among the three electrolytes is within 0.003. Figure 1(b) illustrates the comparison of CE test results from three different testing devices with varying accuracies. For the device with a precision of one part in five thousand, its CE testing fluctuates up to 0.006, making it unable to effectively discern the CE differences brought about by the three electrolytes shown in Figure 1(c). Conversely, devices with a precision of one part in ten thousand or one part in fifty thousand exhibit CE testing fluctuations within 0.001, enabling the effective differentiation of side reactions caused by different electrolytes. This allows researchers to rapidly predict the lithium ion battery lifespan after a few short cycles (around 16 cycles), compared to traditional methods, enabling quick predictions of long-term cycle life (around 500 cycles).
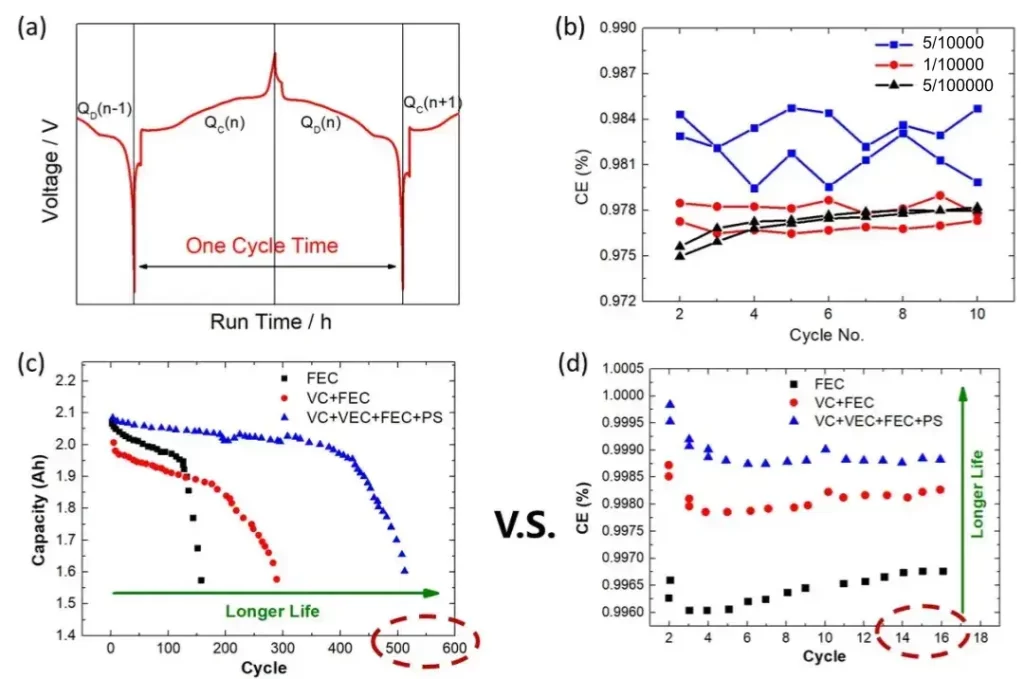
Figure 1. (a) illustrates the charge-discharge curve of lithium batteries; (b) displays the comparison of CE test results from devices with different testing accuracies; (c) and (d) respectively depict the comparison of long-cycle capacities and early-cycle CE comparison results of batteries prepared using three different electrolytes.
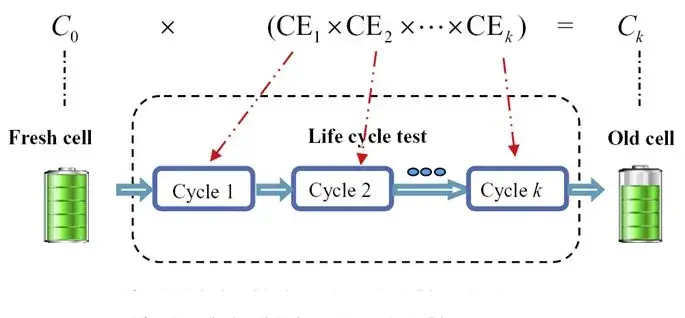
Figure 2. Battery Coulomb Efficiency (CE) and lithium ion battery lifespan Prediction Model [1]。
3. Internal Side Reaction Assessment of Batteries-Material & Electrolyte & Operating Condition Evaluation
Next, let’s introduce the second significance of high-precision current & voltage testing: internal side reaction assessment of batteries. Before delving into detailed application cases, we need to introduce two concepts: ΔC (Charge Endpoint Slippage) or Ch.End.Cap. (%). As shown in Figure 3(a), ΔC can be calculated by subtracting the charging capacity of the previous cycle from that of the subsequent cycle, i.e., ΔC = QC(n+1) – QD(n); while Ch.End.Cap. (%) can be calculated by dividing the charging capacity of the nth cycle by that of the first cycle, i.e., Ch.End.Cap. (%) = QC(n) / QC(1) * 100%. Although these two parameters have different calculation methods, they represent the same significance, both of which can characterize the degree of oxidation reaction occurring in the electrolyte on the positive electrode side. This oxidation reaction continuously consumes the electrolyte and deposits reaction by-products on the surface of the negative electrode material. Over time, this will clog the gaps in the negative electrode material and lead to a drop in battery capacity [2,3]. The specific reaction process is illustrated in Figures 3(c) and (d). Figure 3(b) shows the charge endpoint shift of the battery under multiple cycle conditions, indicating that the positive electrode side continuously consumes active lithium in the electrolyte, gradually affecting the battery’s cycle life. Generally, in a stable cycling process, the values of ΔC or Ch.End.Cap. (%) for mature batteries are relatively small. If the testing accuracy is too low, accurate and effective analysis results cannot be obtained. Therefore, we need high-precision testing equipment for detailed analysis of battery side reactions.
Figure 3(e) also illustrates four aspects of the application of parameters ΔC or Ch.End.Cap. (%): ① Screening of different electrolyte additives; ② Screening of different cathode electrode materials; ③ Determination of oxidation charge under different potentials; and ④ Study of related material mechanisms. Figures 4(a-c) show the comparison of cycle life of LCO batteries under three different electrolytes[4]. Figure 4(d) extracts the Ch.End.Cap. (%) of the first 16 cycles for comparison, and it is found that the electrolytes with the addition of 1wt% or 2wt% VC have much lower Ch.End.Cap. (%) values compared to the electrolyte without VC addition.
This indicates that the addition of VC can slow down the oxidation rate of the electrolyte on the cathode electrode side, thereby extending the battery’s cycle life. From the long-term cycling results shown in Figure 4(e), it can also be seen that after cycling for 110 cycles, the capacity retention rate of the electrolyte without VC addition has dropped to around 86%, while the capacity retention rate of the electrolytes with the addition of 1wt% or 2wt% VC remains above 94%.
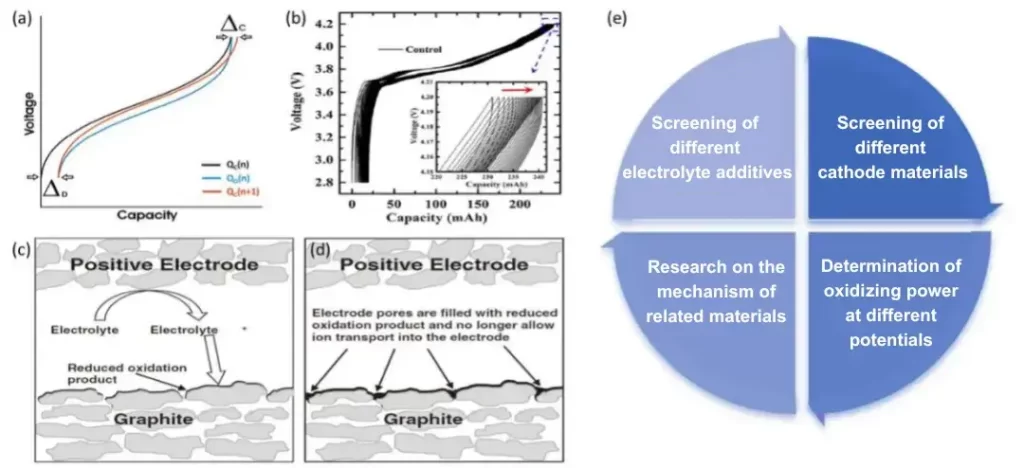
Figure 3. (a) shows the definition of ΔC, which is the charging capacity of the latter lap minus the charging capacity of the former lap, and the formula can be expressed as ΔC = QC(n+1)-QD(n); (b) shows the end-of-charge shift of the battery under a multi-lap cycling; (c) and (d) show the oxidation reaction of the electrolyte on the positive side and deposition of the by-reaction products on the surface of the negative electrode material; and (e) lists the four applications of the parameter ΔC or Ch.End.Cap.(%).
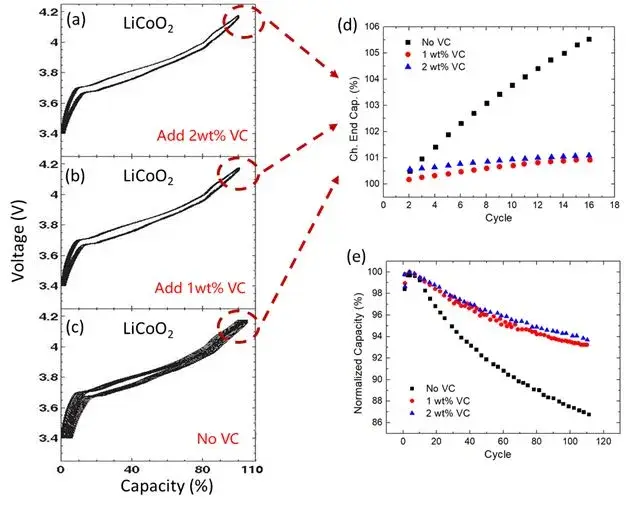
Figures 4. (a-c) demonstrate the comparison of cycle life of LCO batteries under three different electrolytes. (d) extracts the Ch.End.Cap. (%) of the first 16 cycles for comparison. (e) shows the comparison of long-term cycle life of batteries prepared with three different electrolytes.
4. Analysis of Battery Capacity Decay Factors – Fine Analysis of Cell Failure
The third significance of high-precision current & voltage testing lies in the fine analysis of cell failure when utilizing dQ/dV curves (also known as incremental capacity curves or IC curves) or dV/dQ curves (also known as voltage difference curves or DV curves) for capacity decay factor analysis. High-precision testing equipment can assist in analyzing smaller phase transition peaks or detecting weak side reactions, thereby obtaining more refined failure analysis results.
Figure 5(a) illustrates the dQ/dV curve of an LFP battery [5]. The total area under this curve represents the total capacity of the battery, while the area under each peak corresponds to the amount of charge involved in the phase transition process. Additionally, it can be observed that the dQ/dV curve has three distinct peaks, indicating the presence of three significant phase transitions throughout the charging process. These peaks can be labeled as peaks ⑤*II, ②*II, and ①*II, respectively.
Figure 5(b) displays the corresponding dV/dQ curve of the battery, from which we can also clearly delineate three regions. The width of each region corresponds to the battery’s capacity within the respective interval, labeled as QA, QB, and QC. It can be observed that QA, QB, and QC correspond to the areas under the peaks labeled as ⑤*II, ②*II, and ①*II in the dQ/dV curve (as shown in Figure 5(a)). By carefully analyzing parameters such as the shape, position, and area of each peak in the dQ/dV or dV/dQ curves, we can deduce the capacity decay mechanism of the battery, such as active material loss, lithium loss, and so on.
For example, Figure 5(c) shows a comparison of dQ/dV curves after different cycles. The shapes and areas of peaks ⑤*Ⅱ and ②*Ⅱ remain largely unchanged, indicating no significant loss of active material in the battery after cycling. However, peak ①*Ⅱ exhibits a noticeable decrease in peak height, primarily due to the loss of active lithium. Additionally, there is no significant shift in the positions of the peaks in the dQ/dV curves, indicating that there is no significant increase in internal resistance of the battery after long cycling periods.
Figure 5(d) illustrates the difference in testing dQ/dV curves between devices with accuracy of one part per thousand and five parts per thousand. It can be observed that the dQ/dV curves obtained from the device with five parts per thousand accuracy exhibit a higher signal-to-noise ratio, with some small phase transition peaks submerged within the testing fluctuations of the device. This makes it difficult for researchers to obtain effective analysis results. In contrast, the dQ/dV curves obtained from the device with one part per thousand accuracy are much smoother and can capture small phase transition peaks effectively. This assists researchers in quickly identifying early side reactions and makes cell failure analysis more refined!
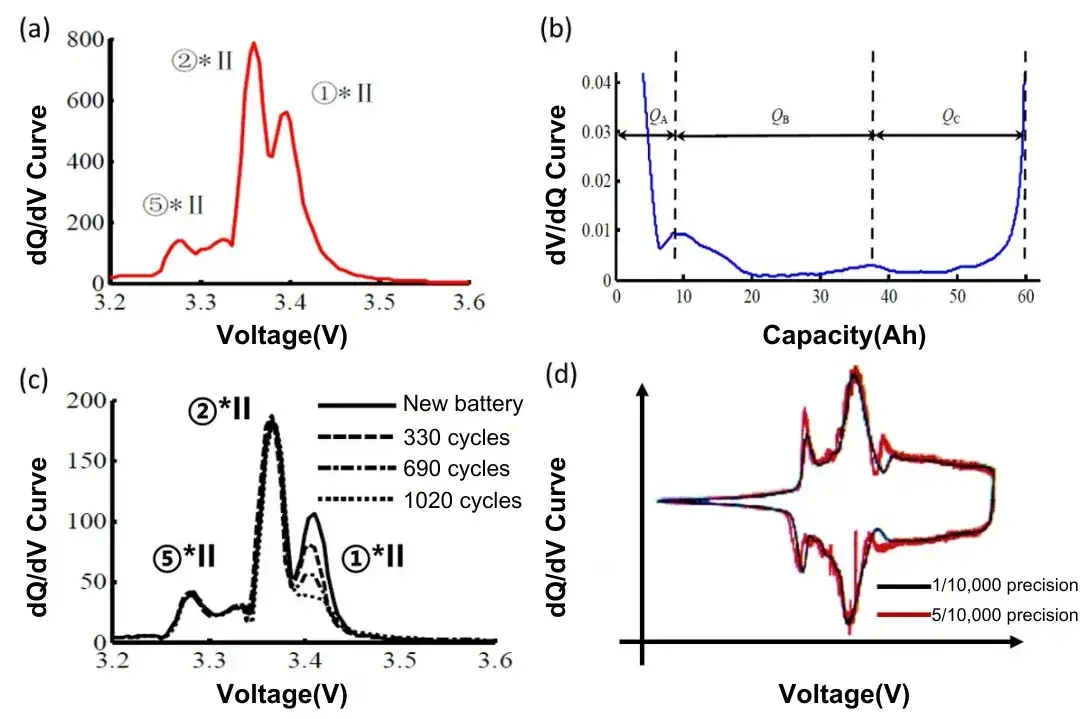
Figure 5. (a) displays the dQ/dV curve of an LFP battery; (b) shows the dV/dQ curve of the same LFP battery; (c) compares dQ/dV curves after different cycles; and (d) presents a comparison of dQ/dV curves obtained from devices with different accuracies.
Since we understand the significant importance of “high-precision” charge-discharge testing equipment with an accuracy of one part per thousand or better, how should we choose such testing devices? Here, we’d like to highlight the Electrochemical Performance Analyzer (ECT & ERT series) developed independently by IEST, as depicted in Figures 6(a-b). This equipment is equipped with eight testing channels with an accuracy of one part per thousand, facilitating researchers in conducting failure analysis and rapid lithium ion battery lifespan prediction as mentioned above. Additionally, the ERT7008 series of this device integrates electrochemical modules such as CV (Cyclic Voltammetry) and EIS (Electrochemical Impedance Spectroscopy), as shown in Figure 6(c). It enables users to incorporate CV or EIS testing steps synchronously into the cycling test steps, facilitating long-term CV or EIS monitoring during cycling tests, thereby addressing the inconvenience of users switching testing equipment back and forth or frequently moving batteries.
Figure 6. Schematic diagram of the high-precision Electrochemical Performance Analyzer (ECT & ERT series) independently developed by IEST, along with the demonstration of CV & EIS functions in the software.
5. References
[1] F.F. Yang, X.B. Song, G.Z. Dong and K.L. Tsui, A coulombic efficiency-based model for prognostics and health estimation of lithium-ion batteries. Energy 171 (2019) 1173-1182.
[2] J.C. Burns, A. Kassam, N.N. Sinha, L.E. Downie, L. Solnickova, B.W. Way and J.R. Dahn, Predicting and Extending the Lifetime of Li-Ion Batteries. Journal of The Electrochemical Society 160 (2013) A1451-A1456.
[3] D.Y.H. Wang, N.N. Sinha, R. Petibon, J.C. Burns and J.R. Dahn, A systematic study of well-known electrolyte additives in LiCoO2/graphite pouch cells. Journal of Power Sources 251 (2014) 311-318.
[4] J.C. Burns, N.N. Sinha, D.J. Coyle, G. Jain, C.M. VanElzen, W.M. Lamanna, A. Xiao, E. Scott, J.P. Gardner and J.R. Dahn, The Impact of Varying the Concentration of Vinylene Carbonate Electrolyte Additive in Wound Li-Ion Cells. Journal of The Electrochemical Society 159 (2012) A85-A90.
[5] X.B. Han,《Research on Mechanistic Model and State Estimation of Automotive Lithium-ion Batteries》, 2014.10.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.