-
iestinstrument
Prussian Blue and Hard Carbon: Powder Conductivity and Compaction Density for Sodium-ion Cathode Materials
1. Preface
Sodium, which shares similar chemical properties with lithium due to their same group placement in the periodic table, offers significant advantages in terms of natural abundance and cost. Sodium-ion batteries (SIBs) have attracted considerable attention as promising alternatives to lithium-ion batteries, owing to their rapid charging capability, superior low-temperature performance, enhanced safety, and compatibility with existing lithium battery manufacturing processes. These attributes position SIBs as strong candidates for the next generation of commercial energy storage systems.
Advances in sodium-ion battery research have led to notable progress in both cathode and anode materials. Cathode materials primarily include layered oxides, polyanionic compounds, Prussian blue analogues (PBAs), and organic compounds. Anode materials are largely categorized into carbon-based materials, titanium-based compounds, organic electrodes, and alloy-based systems.
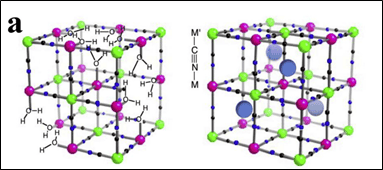
Among these, Prussian blue (PB) and its analogues—representative metal-organic frameworks (MOFs)—have gained traction as cathodes due to their low cost, facile synthesis, and open framework structure. PB-derived nanomaterials retain high surface area, interconnected pores, and tunable pore size distributions, facilitating efficient charge transfer in energy storage systems. By optimizing synthesis conditions such as temperature and atmosphere, PBAs with desirable structural and electrochemical properties can be achieved[1]. Figure 1 illustrates the Prussian blue structure, and Figure 2 shows SEM images of Prussian blue and its derivatives.
 |
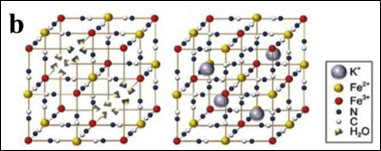 |
Figure 1. Crystal Structure of Prussian Blue and Its Analogues
Figure 2. SEM images of Prussian blue and its derivatives: (a)Na0.67Ni0.33Mn0.67O2
(b)Na0.67Ni0.33Mn0.66Sn0.01O2
(c) Na0.67Ni0.33Mn0.64Sn0.03O2
(d) Na0.67Ni0.33Mn0.62Sn0.05O2[2]
On the anode side, carbon-based materials—particularly hard carbon—are considered the most practical choice due to their low sodium insertion potential, high capacity, excellent cycling stability, resource availability, and simple preparation process. Hard carbon stands out for its large interlayer spacing, low cost, tunable synthesis, and the possibility of deriving from renewable precursors. Figure 3 illustrates a typical hard carbon synthesis process and its microstructural characteristics.
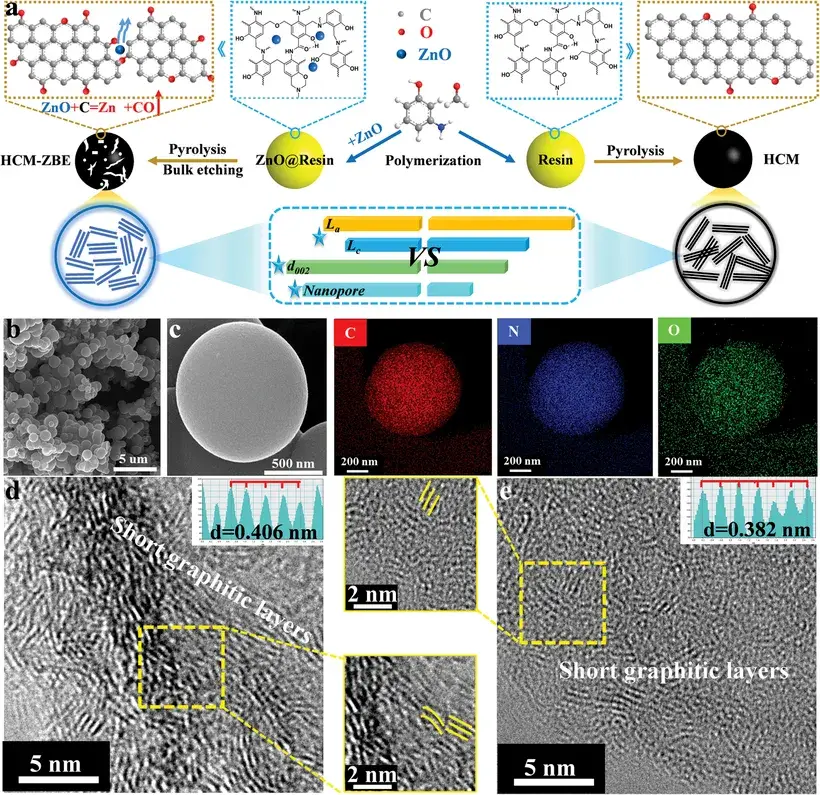
Figure 3. Schematic diagram of hard carbon synthesis and microstructure characterization diagram.
Evaluating prussian blue structure, electronic conductivity and prussian blue density together with hard carbon density and conductivity at the powder stage is critical for rapid material screening and downstream electrode design.
So, in this study, four types of Prussian blue (PB-1 to PB-4) and four types of hard carbon (HC-1 to HC-4) were evaluated using the PRCD3100 system to measure their electrical conductivity and compaction density under various pressure conditions.
2. Test Method
The PRCD3100 (IEST) was used to characterize the conductivity and Prussian blue density and hard carbon density properties. The Prussian blue samples(PB-1/PB-2/PB-3/PB-4) were tested in a two-probe mode, while the hard carbon samples(HC-1/HC-2/HC-3/ HC-4) were measured using a four-probe method to improve accuracy. The testing equipment is shown in Figure 4.
Test parameters included an applied pressure range of 10–200 MPa, incremented at 20 MPa intervals, with a 10-second hold at each pressure step.
Figure 4. (a) Appearance of PRCD3100; (b) Structure of PRCD3100
3. Test Results and Analysis
3.1 Prussian blue materials
Prussian blue and its analogues feature a three-dimensional open framework that enables efficient sodium ion intercalation and deintercalation, making them ideal cathode materials for SIBs. Although PBAs offer a theoretical specific capacity of 170 mAh/g and good cycling stability, practical application has been limited by issues such as structural vacancies, coordinated water molecules, and poor rate capability. These factors reduce specific capacity, impede ionic conductivity, and can lead to structural collapse during cycling.
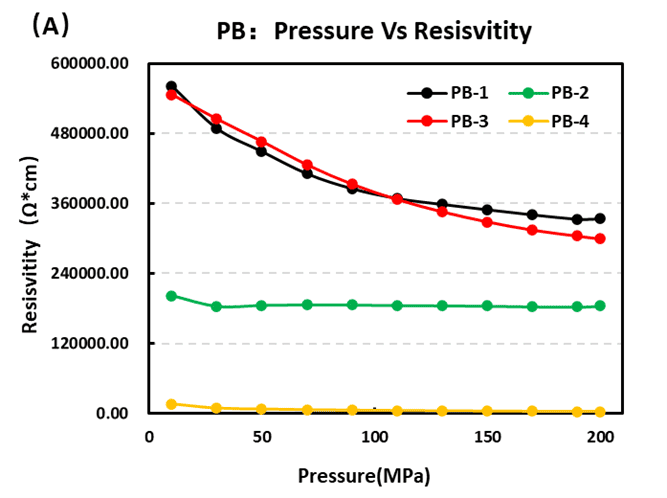
Modification strategies have been developed to enhance the physical and electrochemical properties of PBAs. Figure 5 presents the resistivity and conductivity results for the four Prussian blue samples. PB-2 and PB-4 are modified versions of PB-1 and PB-3, respectively. The results clearly show that PB-2 and PB-4 exhibit superior conductivity, confirming that material modifications can effectively improve electronic transport.
 |
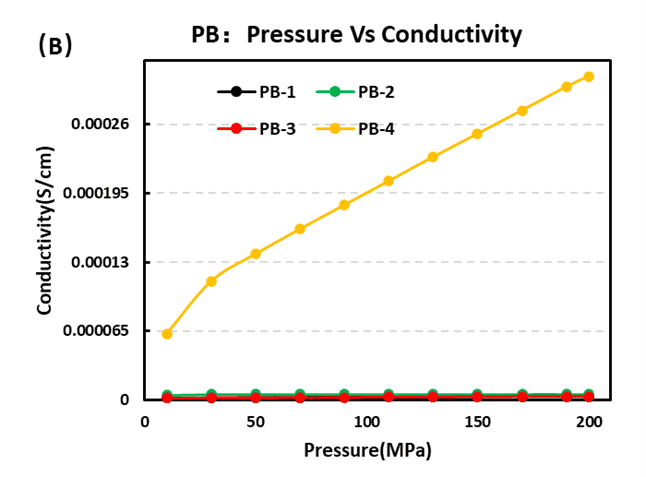 |
Figure 5. (A) Resistivity and (B) Conductivity of Four Prussian Blue Materials
Compaction testing revealed variability in prussian blue density across samples. Measured compaction densities followed PB-1 > PB-3 > PB-4 > PB-2 under the current preparation and pressing conditions. This indicates that higher intrinsic particle packing (or lower porosity) does not always correlate directly with conductivity improvements; thus, prussian blue structure and surface chemistry must be considered alongside density when screening cathode powders.
Key takeaways for PB materials:
-
Modified PBs can deliver better conductivity but may alter packing behavior.
-
Powder-level conductivity testing helps identify promising PB derivatives before electrode fabrication.
-
Optimal PB performance requires balancing crystal vacancies, water content and conductive additives.
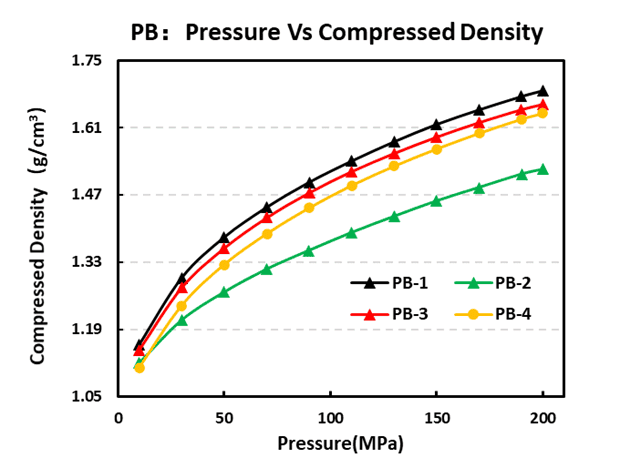
Figure 6. Compaction density test results for four Prussian blue materials.
3.2 Hard carbon materials
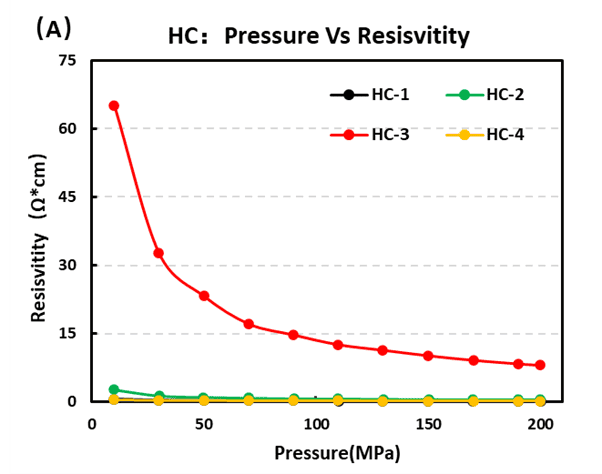
Hard carbon is the leading negative-electrode choice for Na-ion cells. Our four-probe resistivity tests rank conductivity as HC-1 > HC-4 > HC-2 > HC-3, with HC-1 showing the best electronic transport. Compaction testing returned hard carbon density order HC-4 > HC-1 > HC-2 > HC-3, indicating that HC-4 packs most densely under identical pressing conditions.
Compression–rebound profiling highlights differences in mechanical behavior that affect electrode calendering and porosity control. Materials with favorable combinations of low resistivity and high compaction density (e.g., HC-1 and HC-4) are promising candidates for high-performance anodes, but selection must consider trade-offs between compressibility, elastic rebound and particle fracture.
 |
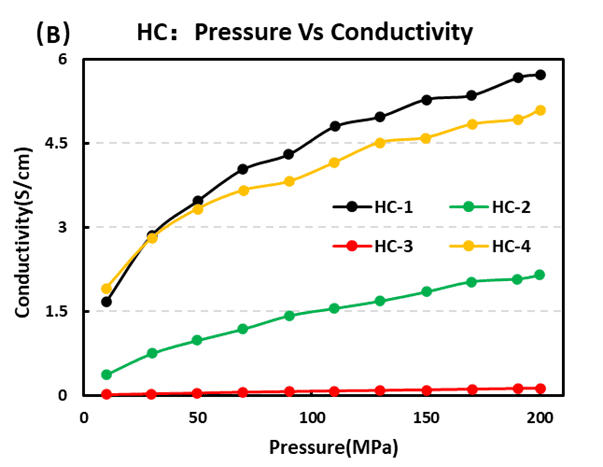 |
Figure 7. (A) Resistivity and (B) Conductivity of Four Hard Carbon Materials
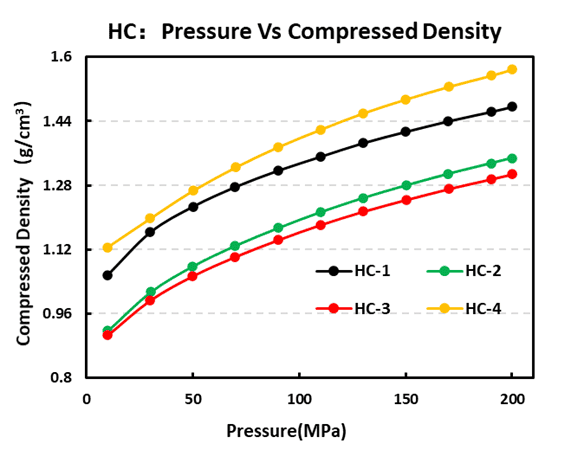
Figure 8. Compaction density test results of four hard carbon materials
4. Discussion: Linking powder metrics to electrode performance
Powder resistivity and compaction density are rapid, low-cost diagnostics that predict key electrode attributes:
-
Electronic percolation: Low powder resistivity suggests fewer conductive fillers are required to reach target electrode conductivity.
-
Volumetric energy density: Higher compaction density often translates to higher volumetric capacity at the electrode level.
-
Processing window: Compression and rebound curves inform calendaring pressure limits to avoid particle damage while maximizing density.
For PB cathodes, modifying the prussian blue structure to reduce coordinated water and stabilize vacancies can improve conductivity but may reduce compaction if particle morphology changes. For hard carbon, tuning microstructure and graphitic domains optimizes both hard carbon density and sodium storage kinetics.
4. Summary
This study utilized the PRCD3100 powder resistance and compaction density system to evaluate the conductivity and density properties of Prussian blue cathode and hard carbon anode materials for sodium-ion batteries. The results clearly differentiate the performance among various samples, demonstrating that this method is an effective tool for rapid and reliable material screening. These insights can guide further optimization of sodium-ion battery materials toward higher performance and commercial viability.
5. References
[1] Chen J, Wei L, Mahmood A, et al. Prussian blue, its analogues and their derived materials for electrochemical energy storage and conversion – ScienceDirect[J]. Energy Storage Materials, 2020, 25:585-612.
[2] Li J, Risthaus T, Wang J, et al. The effect of Sn substitution on the structure and oxygen activity of Na0.67Ni0.33Mn0.67O2 cathode materials for sodium ion batteries[J]. Journal of Power Sources, 2019, 449:227554.
[3] Yin X, Lu Z, Wang J, et al. Enabling Fast Na+ Transfer Kinetics in the Whole-Voltage-Region of Hard-Carbon Anodes for Ultrahigh-Rate Sodium Storage[J]. Advanced Materials, 2022.
[4] Wu Junda, Zhao Yabin, Zhang Fuming. Research progress on hard carbon materials as anode materials for room temperature sodium-ion batteries [J]. Shandong Chemical Industry, 2019, 488.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


