-
iestinstrument
Solid State Electrolyte Powder Ionic Conductivity Testing
1. Background
Solid state electrolyte is a type of material capable of ion conduction in the solid state, playing a crucial role in the performance of all-solid-state lithium-ion batteries. Compared to traditional liquid-state lithium-ion batteries, solid-state batteries feature electrolytes composed entirely of solid materials, replacing conventional PP/PE separators. This substitution significantly reduces the risks associated with lithium dendrite growth and other side reaction byproducts piercing the separator, which are typically caused by negative electrode side reactions and lithium deposition. As a result, the safety of the battery is significantly enhanced.
Currently, solid state electrolytes can be classified into several main categories based on their chemical composition and physical properties: oxides, sulfides, polymers, and halides. Oxide solid electrolytes exhibit high hardness and brittleness and typically require combination with liquid electrolytes, known as semi-solid-state batteries.
Sulfide solid state electrolytes possess good processability, but due to their reactivity with moisture in the air, they demand stringent manufacturing conditions (dry room dew point requirement below -60°C). However, due to their soft texture and high conductivity, sulfide-based solid-state batteries hold the most promise if stability issues can be effectively addressed. Polymer solid electrolytes have lower conductivity and electrochemical windows, resulting in poorer rate capability. Halide solid electrolytes offer excellent ion conductivity and high-voltage stability, making them suitable for high-energy-density applications. However, they are currently in the experimental research and development stage [1].
The core difference between solid-state batteries and liquid-state batteries lies in their electrolyte composition and form. The electrolyte of a liquid-state battery is primarily composed of carbonate solvents combined with lithium salts to form a liquid electrolyte. In contrast, the electrolyte of a solid-state battery consists of solid materials.
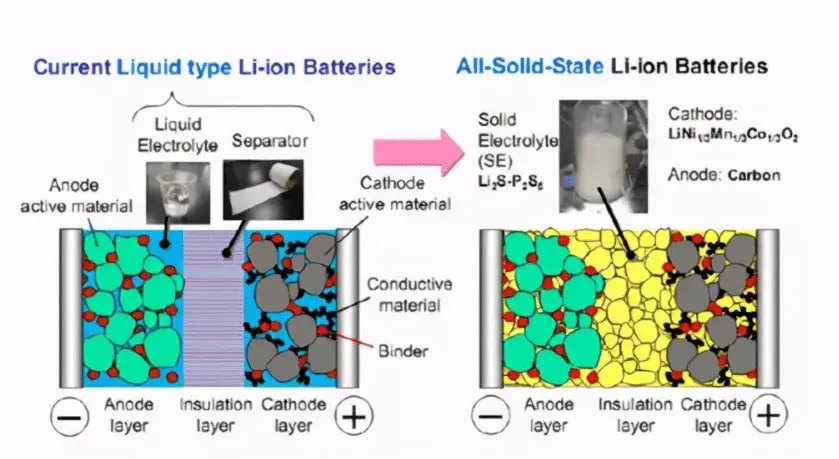
Figure 1. Differences between Liquid-State and Solid-State Batteries [1]
In the development path of solid state electrolytes, Japan and South Korea mainly pursue the sulfide route. Particularly in Japan, extensive national efforts and years of deep research have positioned the country at the forefront of solid-state battery technology worldwide. Meanwhile, domestic enterprises in China primarily focus on the oxide route, adopting a semi-solid-state technical approach for faster industrialization. However, influenced by the development trends in Japan and South Korea and guided by domestic policies, an increasing number of Chinese companies are now joining the competition in the sulfide all-solid-state battery route. Key players include leading lithium-ion battery companies such as CATL and BYD, established battery firms like EVE Energy and Fengli, as well as startups such as JW Energy, CBAK Energy, Gotion High-Tech, Beijing National Battery Technology, Zhongke Gu’neng, and Zhongke Shenlan Huize, among others.
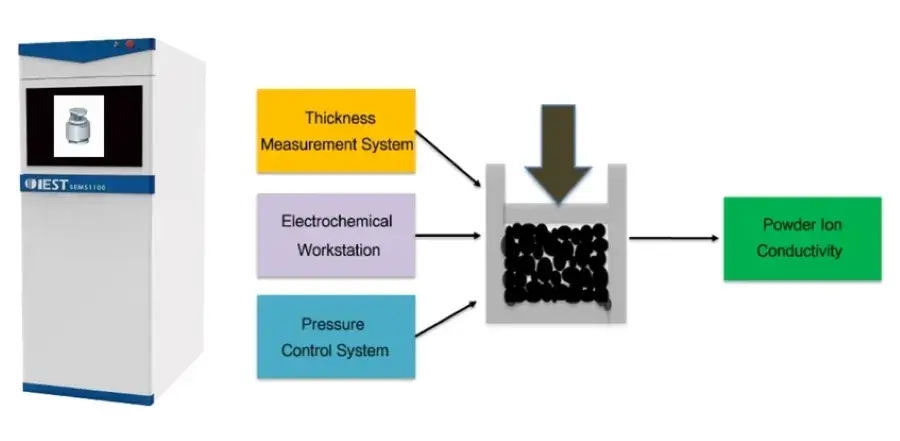
Pressure significantly influences the electrochemical properties of solid state electrolytes. Under high pressure, the contact between solid-solid interfaces of solid electrolytes is enhanced, aiding in the improvement of ion conductivity and thereby enhancing the overall performance of solid-state batteries. In support of the development of the all-solid-state battery industry, IEST has upgraded traditional powder resistance and compaction density meters, introducing the SEMS1100 Solid Electrolyte Measurement System. This system enables real-time testing of solid state electrolyte ion conductivity under pressure.
Figure 2. SEMS1100 Instrument: Physical Appearance and Working Principle
Built upon the functionalities of powder resistance and compaction testers, the SEMS1100 system features expanded capabilities. Equipped with specially developed sealing molds and heating modules by IEST, it enables precise testing of powder ion conductivity variations under different temperatures and pressures.
2. Experimental Section
2.1 Test Equipment
Testing Equipment: Solid electrolyte testing system SEMS1100 paired with Donghua Electrochemical Workstation DH7001;
Testing Conditions: Pressurization mode of 50-350 MPa with a pressure holding time of 180 min; EIS test frequency range of 1 MHz-0.1 Hz, perturbation amplitude of 10 mV;
Test Sample 1: Sulfide solid electrolyte LPSC, sample quantity 0.15 g, using φ13 mm ceramic-sealed mold;
Test Sample 2: Oxide solid electrolyte LLZO powder, sample quantity 0.1 g, using φ13 mm ceramic-sealed mold.
3. Test Results
3.1 Test Results of LPSC Powder
As we all know, sulfide LPSC is a solid state electrolyte that currently rivals organic solvent electrolytes in terms of conductivity. The ion conductivity of LPSC is influenced by factors such as raw material particle size, sintering temperature, and sintering time during the preparation process [3]. Additionally, during testing, environmental temperature and pressure during powder compression also have a significant impact on the test results of solid state electrolytes [4].
Based on this, IEST used the SEMS1100 to test the ion conductivity of LPSC under different pressures. From the EIS spectra of LPSC powder under pressure, it can be observed that as the pressure increases, the EIS gradually decreases. The reduction in powder EIS indicates an enhancement in ion conductivity. The main reason for this phenomenon is that with increasing pressure, the compaction density of the solid state electrolyte increases, leading to a corresponding decrease in the porosity of the solid electrolyte pellets. This results in a tighter contact between particles, reducing the ion transport resistance at grain boundaries, thereby enhancing the ion transport capability and causing a decrease in EIS [5,6].
From the figure and the table data, it can be observed that the SEMS1100 can accurately control the pressure applied to the solid state electrolyte during compression. We can monitor the change in sample thickness in real time during the testing process. Combining the EIS data, we can accurately understand the variation of ion conductivity during the compression process.
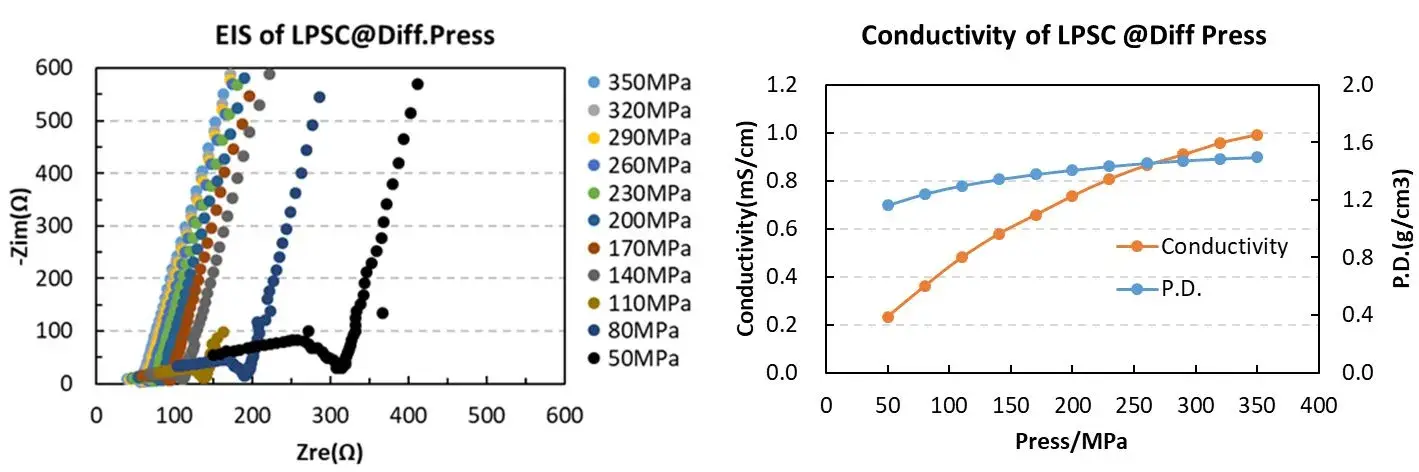
Figure 3. EIS Spectra and Ion Conductivity Variation of LPSC Powder
Table 1. EIS Test Data of LPSC Powder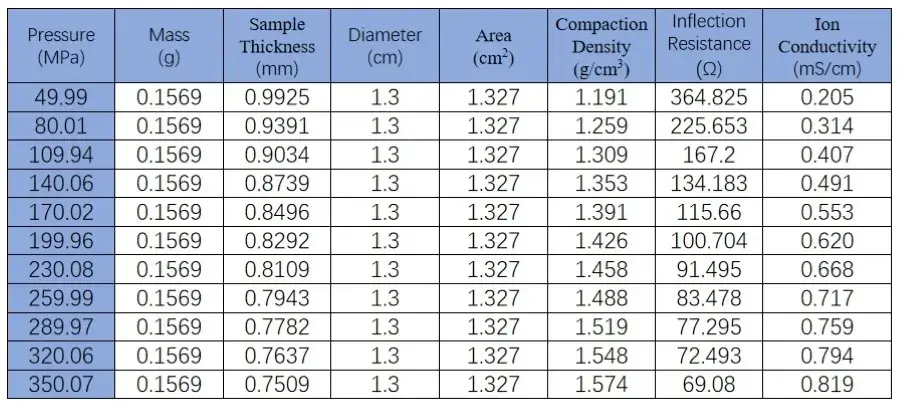
3.2 Test Results of LLZO Oxide Powder
LLZO, as a garnet-type structure oxide solid electrolyte, typically exhibits a conductivity of 0.1 to 0.9 mS/cm after sintering, which is lower than that of organic solvent electrolytes. For LLZO powder itself, the diffusion rate of lithium ions at grain boundaries is much lower than that within the crystal bulk phase, and the number of grain boundaries and particle size have a significant impact on ion conductivity [7].
IEST conducted tests on the ion conductivity of LLZO powder under different pressures using the SEMS1100. From the EIS spectra of LLZO powder under pressure, it can be observed that at low pressures, the EIS curve of LLZO powder appears more chaotic, especially in the intermediate frequency region, where the EIS curve is disorderly. As the pressure increases, the EIS of LLZO powder decreases significantly. Although the EIS curve remains somewhat chaotic in the intermediate frequency region, it is generally complete. The main reason for this is that with increasing pressure, the compaction density of the solid state electrolyte improves, leading to better contact between particles and reduced ion transport resistance at grain boundaries.
From the figure and the table data, it can be observed that although the ion conductivity of LLZO increases somewhat under higher pressure, it remains relatively low, reaching only 2.14*10-5 mS/cm, far below the actual requirements for battery usage. Therefore, in practical applications, LLZO solid electrolytes are mainly used in the form of powder coating, applied onto separators or the surfaces of positive and negative electrodes. They often need to be combined with electrolytes or polymers to achieve the required performance for lithium-ion batteries and meet the practical demands of battery usage.
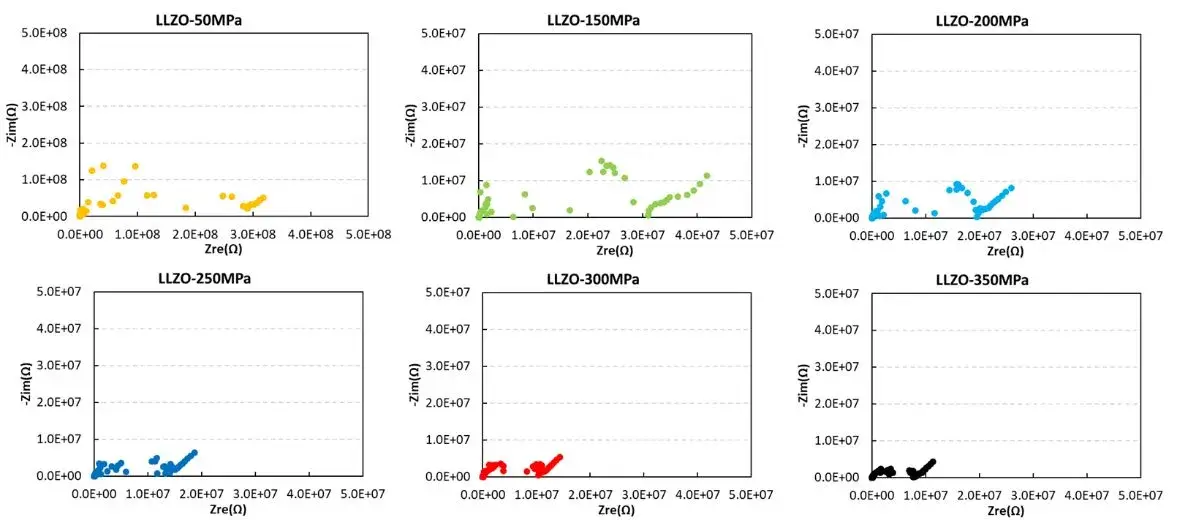
Figure 4. EIS Spectra and Ion Conductivity Variation of LLZO Powder
Table 2. EIS Test Data of LLZO Powder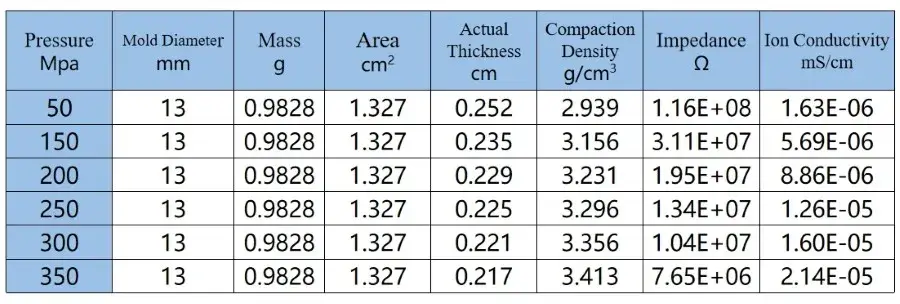
4. Conclusion
By using the SEMS1100 in conjunction with the electrochemical workstation, we can accurately test the solid state electrolyte powder ion conductivity under actual pressure conditions. Additionally, we can monitor the changes in sample thickness in real time under pressure, allowing us to calculate the compaction variation.
The test results indicate that sulfide solid state electrolytes, due to their softer texture, can still achieve an ion conductivity of 0.9 mS/cm even under pressures exceeding 300 MPa, even with relatively small particle sizes (according to customer feedback, D50 is 1 μm). This conductivity level meets the requirements for charging and discharging of all-solid-state batteries at lower rates. In contrast, oxide solid electrolytes, due to their lower intrinsic ion conductivity and higher hardness, exhibit poorer contact between particles and grain boundaries, particularly evident under low pressure conditions, as reflected in chaotic EIS spectra. Even under high pressure conditions, significant fluctuations are observed in the EIS spectra in the intermediate frequency region, and their ion conductivity is low, reaching only 2.14*10-5 mS/cm, far below the actual requirements for battery usage [7,8].
In summary, oxide solid state electrolytes typically exhibit low ion conductivity in practical applications and often require combination with polymers or liquid electrolytes. In contrast, sulfide solid state electrolytes can achieve higher ion conductivity after compression and do not require sintering into pellets or combination with polymers or liquid electrolytes. Therefore, sulfide all-solid-state batteries represent the most competitive route. However, challenges such as poor air stability, weak interface stability at the negative electrode, poor solvent compatibility, and reduced ion conductivity after nanostructuring still exist [9,10]. These issues require further efforts from industry professionals to resolve. We firmly believe that, with the relentless efforts of numerous researchers, the various challenges of solid-state batteries will gradually be overcome and eventually become part of our daily lives.
5. References
[1] New Material Series Report (I): Solid State Battery Potential Verified, Focus on New Demand for Power Battery Metals, Guotou Securities, January 24, 2024;
[2] Zhang Fangnan, Hive Energy Solid State Battery Focuses on Sulfide Route, Power Battery Sub-Forum of China Electric Vehicle Hundred, March 17, 2024
[3] Jianming Tao.,Unraveling the performance decay of micro-sized silicon anodes in sulfide-based solid-state batteries, Energy Storage Materials,2024
[4] Chanhee Lee, Stack Pressure Measurements to Probe the Evolution of the Lithium-Solid-State Electrolyte Interface, ACS Energy Letters. 2021,
[5] J. Gu, Z. Liang, J. Shi, Y. Yang, Electrochemo-Mechanical Stresses and Their Measurements in Sulfide-Based All‐Solid‐State Batteries: A Review. Advanced Energy Materials, 2022
[6] A. Hayash, N. Masuzawa1, S. Yubuchi, A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature, Nature Communications,2019
[8] Qi,Liu;etc. Challenges and perspectives of garnet solid electrolytes for all solid-state lithium batteries. Journal of Power Sources,2018.
[9] Oh B. Chae, Brett L. Lucht etc. Interfacial Issues and Modification of Solid Electrolyte Interphase for Li Metal Anode in Liquid and Solid Electrolytes, Advanced Energy Meterials,2023,
[10] Gabin Yoon, Sewon Kim, Ju-Sik Kim, Design Strategies for Anodes and Interfaces Toward Practical Solid-State Li-Metal Batteries, Advanced Science
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.