-
iestinstrument
Tailoring Redox Couples of Li-Rich Mn-Based Cathode Materials by In-Situ Surface Reconstruction
Journal: Nano Energy
Affiliation: School of Materials, Xiamen University
Authors: Xutao Zhu, Xujia Xie, Jie Lin*, Yuanyuan Liu, Guiyang Gao, Yong Yang, Yinggan Zhang*, Weicheng Xiong, Yidi Jiang, Qiyuan Li, Dongliang Peng*.
Correspondence author: LIN Jie, ZHANG Ying-Gan, PENG Dong-Liang.
Instruments used: IEST SPFT-2000 Single Particle Mechanical Properties Test System
1. Abstract
Li-rich Mn-based cathodes deliver exceptional specific capacity because they combine cationic and anionic redox activity. However, lattice-oxygen participation at high voltages generates unstable electronic holes and reactive O²⁻ species that ultimately form O–O dimers and release O₂, causing irreversible oxygen loss, cation vacancy formation, structural collapse, and rapid capacity/voltage fade. Here we summarize a recently reported in-situ near-surface reconstruction strategy that introduces near-surface Ni²⁺ and PO₄³⁻ into Li-rich layered oxides and simultaneously forms an amorphous Li₃PO₄ coating. Density functional theory (DFT) and comprehensive electrochemical/structural characterization show that this modification lowers TM-3d/O-2p energy levels, raises the oxygen redox potential, stabilizes lattice oxygen, suppresses gas evolution (O₂, CO₂), and markedly improves cycling, rate performance and high-temperature stability. Key performance improvements include capacity retention increase from 35.9% to 77.0% after 700 cycles at 1 C, and capacity retention at 55 °C rising from 32.1% to 85.9% after 120 cycles at 1 C.
2. Challenges for Li-rich Mn-based Cathode Redox Couples and Lattice Oxygen
Li-rich Mn-based layered oxides (LLOs) exploit both transition-metal redox and lattice-oxygen redox to reach high specific capacities, making them promising candidates for next-generation high-energy batteries. However, when cycled at high cutoff voltages or elevated temperature, lattice oxygen oxidation creates electronic holes and reactive oxygen species (O²⁻ → Oⁿ⁻ / O–O dimers), which can dimerize and evolve as O₂ (bond length <1.5 Å). This irreversible oxygen loss triggers Li de-intercalation, transition-metal migration, cation vacancy clustering and layered-to-spinel/rocksalt phase transformation—processes that accelerate capacity and voltage decay. Moreover, an unstable cathode–electrolyte interface (CEI), electrolyte oxidation and stress accumulation further degrade particle integrity and kinetics. A unified design guideline for tuning LLO redox couples and stabilizing lattice oxygen has remained elusive.
A recent study from Xiamen University, led by Professors Peng Dongliang, Lin Jie, and Zhang Yinggan, presents an innovative solution. Inspired by high-voltage polyanionic cathodes, the team developed an “in-situ surface reconstruction” strategy involving near-surface co-doping and coating. This approach successfully tailors the redox couples and enhances the structural stability of LLOs. The research results were published in the international academic journal Nano Energy under the title “Tailoring Redox Couples of Li-Rich Mn-Based Cathode Materials by In-Situ Surface Reconstruction for High-Performance Lithium-Ion Batteries“.
3. Design concept: In-situ Surface Reconstruction To Tailor Redox Couples
The reported strategy uses near-surface co-doping (Ni²⁺ and PO₄³⁻) plus formation of an amorphous Li₃PO₄ surface layer (denoted LLO-NP@LPO). DFT calculations indicate that introducing Ni²⁺ and PO₄³⁻ shifts TM-3d/O-2p bands to lower energies: the TM-3d–O-2p center moves (example: from 4.556 eV to 4.028 eV) while nonbonding O-2p states shift markedly (0.065 eV → −1.287 eV). This band lowering raises the oxidation potential for lattice oxygen and reduces the reactivity of nonbonding O-2p orbitals, increasing oxygen redox reversibility. Notably, the O-2p nonbonding shift (≈1.352 eV) exceeds the TM-3d–O-2p shift (≈0.528 eV), indicating greater stabilization of oxygen states and enhanced overlap between O-2p nonbonding orbitals and TM orbitals—factors that favor reversible oxygen redox.
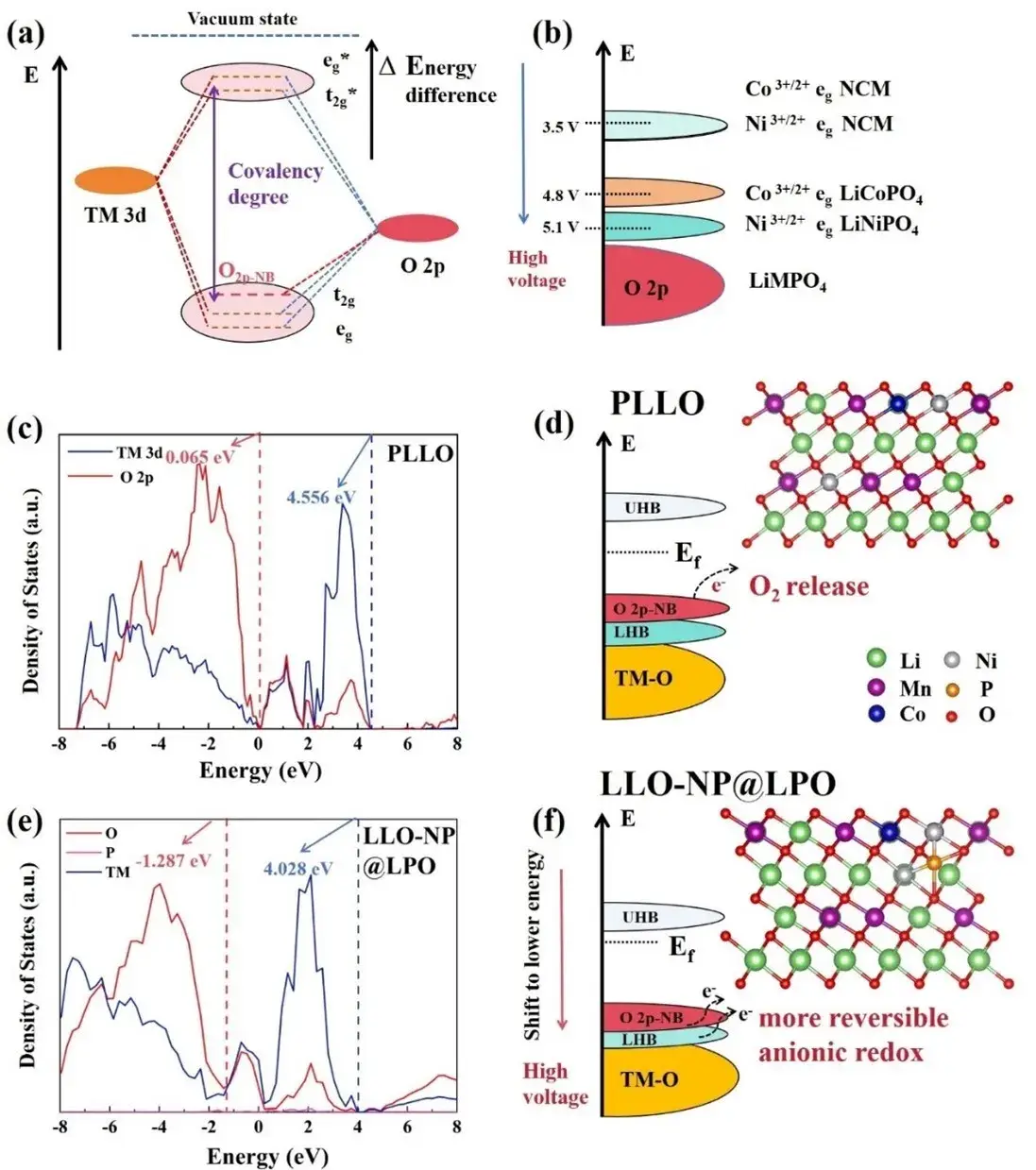
Figure 1. (a) Schematic diagram of molecular orbital energy levels for transition metal 3d orbitals and oxygen 2p orbitals. (b) Energy level diagrams of Ni3+/2+/Co3+/2+ redox pairs in olivine LiMPO4 anode and ternary NCM anode. (c, e) State density maps of O 2p states and TM 3d orbitals in PLLO and LLO-NP@LPO obtained by DFT calculations. (d, f) Electronic structure maps based on the state density maps and schematic diagrams of the two crystal structures used for the calculations.
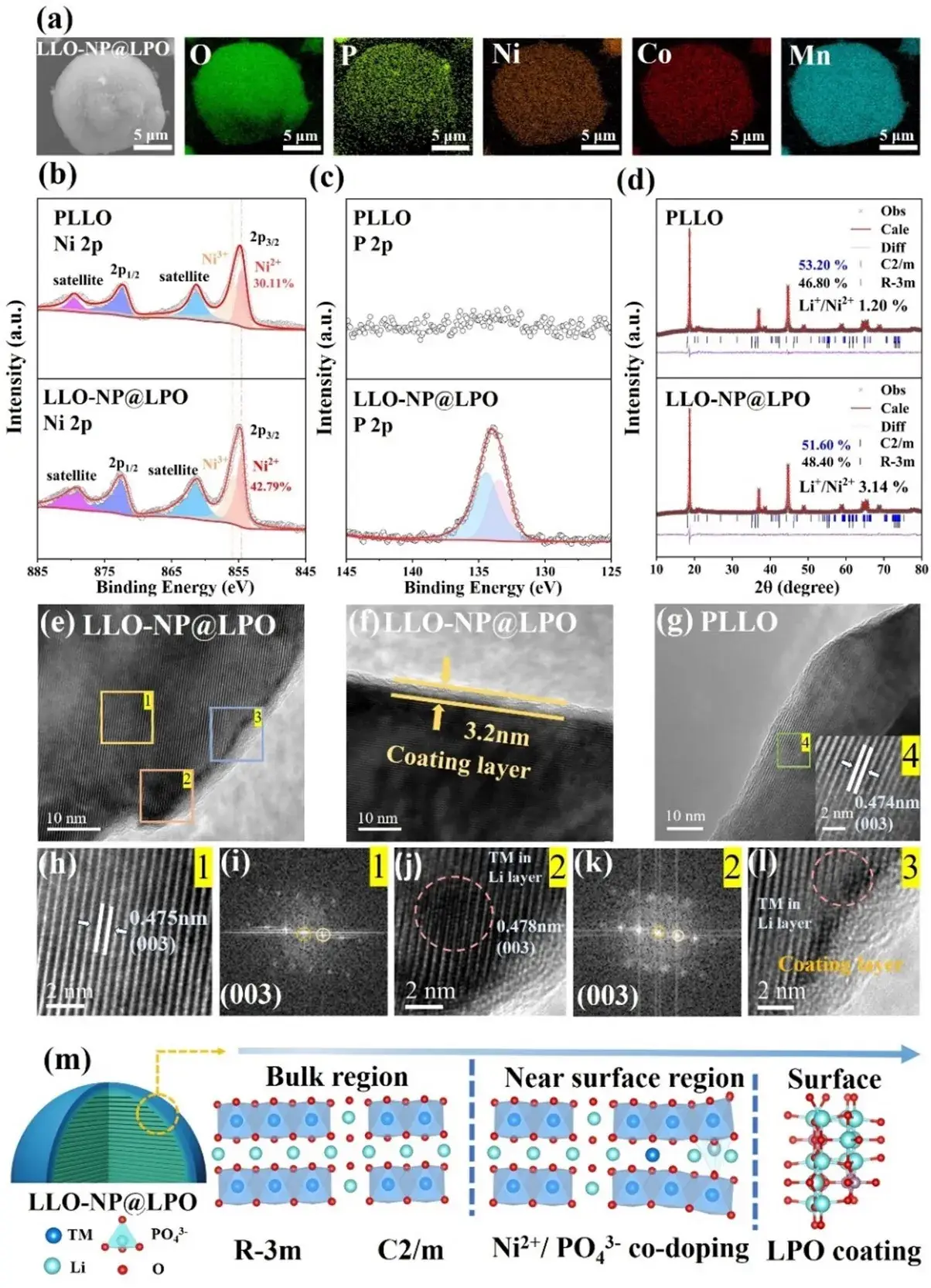
Figure 2. (a) SEM and EDS maps of LLO-NP@LPO. (b, c) XPS maps of Ni 2p and P 2p. (d) XRD Rietveld refinement results of PLLO and LLO-NP@LPO. (e, f) HR-TEM images of LLO-NP@LPO and (g) HR-TEM images of PLLO. (h, j) Enlarged HR-TEM images of regions 1-2 in Fig. (e) and the corresponding Fourier transform (FFT) images in Figs. (i, k). (l) Magnified HR-TEM image of region 3 in Fig. (e). (m) Schematic representation of the near-surface crystal structure of LLO-NP@LPO after in situ surface reconstruction.
4. Structural and Compositional Evidence of Near-surface Reconstruction
Microscopy and spectroscopy confirm the designed near-surface changes and coating:
-
SEM/EDS and XPS show uniform particle morphology and surface distribution of Ni, Co, Mn, O and P. Surface Ni²⁺ fraction rises to 42.79% in LLO-NP@LPO versus 30.11% in PLLO, decreasing toward bulk after etching (200 s), consistent with a gradient, near-surface doping profile.
-
XRD (Rietveld refinement) reveals both R-3m LiTMO₂ and C2/m Li₂MnO₃ phases in both samples. The (003) peak in LLO-NP@LPO shifts to lower angle (slightly increased c-axis), and the (003)/(104) intensity ratio drops from 2.14 (PLLO) to 2.04 (LLO-NP@LPO), indicating modest Li/Ni mixing (<5% recommended) and structural accommodation of PO₄ groups. Li₂MnO₃ fraction decreases from 53.2% to 51.6%, reducing Li–O–Li motifs prone to irreversible oxygen loss.
-
HRTEM shows a ≈3.2 nm amorphous Li₃PO₄ coating and evidence of transition-metal occupation at former lithium sites in the near surface—consistent with the intended reconstruction.
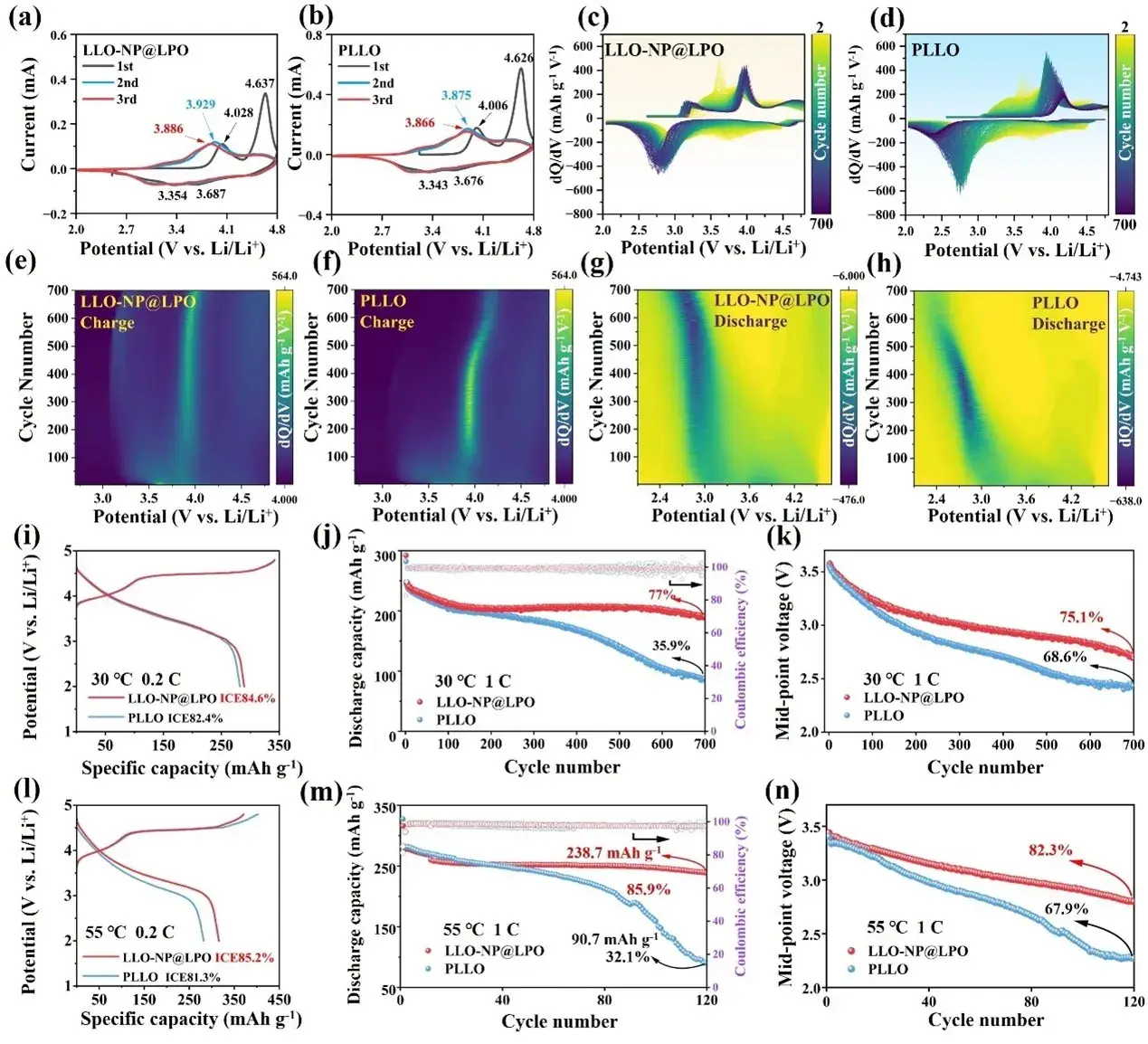
Figure 3. (a-b) Cyclic voltammograms of LLO-NP@LPO and PLLO. (c-d) Cyclic dQ/dV curves and (e-h) corresponding 2D contour plots. initial charge/discharge curves at (i) 0.2C at 30 °C, (j) cyclic performance, and (k) average discharge voltage at 1C. initial charge/discharge curves at (l) 0.2C at 55 °C, (m) cyclic performance, and (n) average discharge voltage at 1C.
5. Electrochemical performance: Improved Redox Reversibility, Cycling and High-temp Stability
Electrochemical probes quantify the benefits to redox couples and lattice oxygen behavior:
-
Cyclic voltammetry: First-cycle oxidation peaks shift upward in LLO-NP@LPO (e.g., 4.028 V and 4.637 V vs. PLLO at 4.006 V and 4.626 V), indicating higher redox potentials for both TM and oxygen redox couples, consistent with DFT.
-
Long-term cycling at 1 C: After 700 cycles, capacity retention improves from 35.9% (PLLO) to 77.0% (LLO-NP@LPO). Average discharge-voltage retention rises from 68.6% to 75.1%; per-cycle voltage fade reduces from 1.58 mV to 1.27 mV.
-
Elevated temperature (55 °C): At 1 C and after 120 cycles, LLO-NP@LPO retains 85.9% capacity (238.7 mAh g⁻¹), whereas PLLO retains only 32.1% (90.7 mAh g⁻¹). Voltage retention at 55 °C improves from 67.9% (PLLO) to 82.3% (LLO-NP@LPO).
-
Rate capability: LLO-NP@LPO shows superior kinetics across multiple C-rates, attributed to enlarged interlayer spacing and the fast-ion Li₃PO₄ surface conductor.
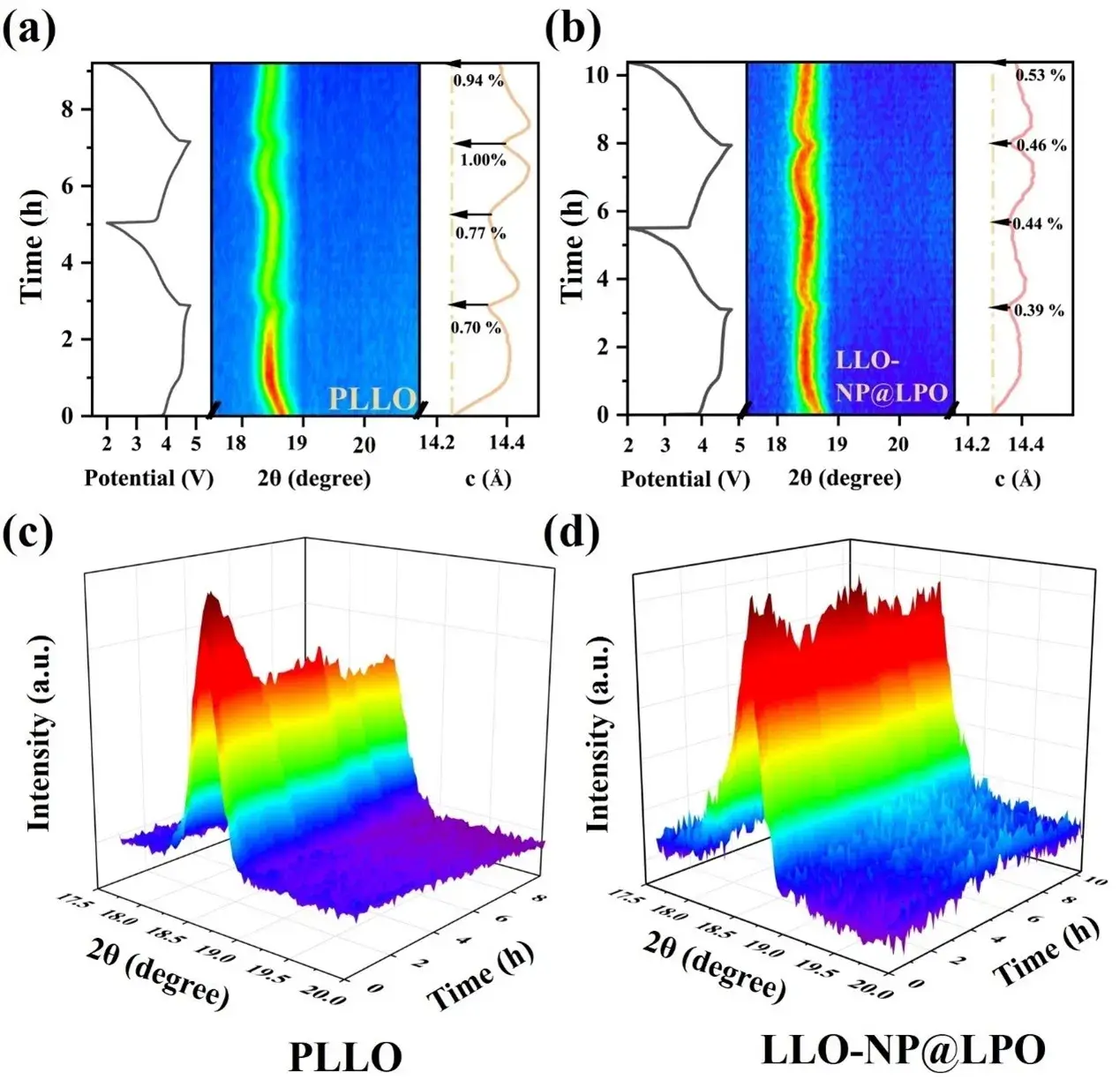
Figure 4. (a) In situ XRD curves of the first two cycles of (a) PLLO and (b) LLO-NP@LPO and the corresponding numerical c-axis curves of charging and discharging. (c) 3D contour plots of the evolution of the (003) peak for the first two cycles of PLLO and (d) LLO-NP@LPO.
6. Probing Gas Evolution, Impedance and Ion Diffusion Related to Lattice Oxygen
Direct and kinetic measurements support reduced oxygen loss and better interfacial stability:
-
DEMS: O₂ release in the first cycle drops from 10.02 µmol g⁻¹ (PLLO) to 6.79 µmol g⁻¹ (LLO-NP@LPO); CO₂ evolution drops from 44.74 to 21.14 µmol g⁻¹, indicating suppressed electrolyte decomposition and carbonate oxidation.
-
EIS: Initial charge-transfer resistances (Rct) are 90.27 Ω (PLLO) and 77.87 Ω (LLO-NP@LPO). After 100 cycles, Rct decreases to 64.05 Ω (PLLO) and 26.09 Ω (LLO-NP@LPO), showing a more stable and lower-resistance electrode/electrolyte interface in the modified sample.
-
GITT/Diffusion: Post-100 cycles, the Li⁺ diffusion coefficient (D_Li⁺) for LLO-NP@LPO remains higher than PLLO at high states of charge where oxygen redox dominates, demonstrating improved ion transport under conditions that typically slow kinetics.
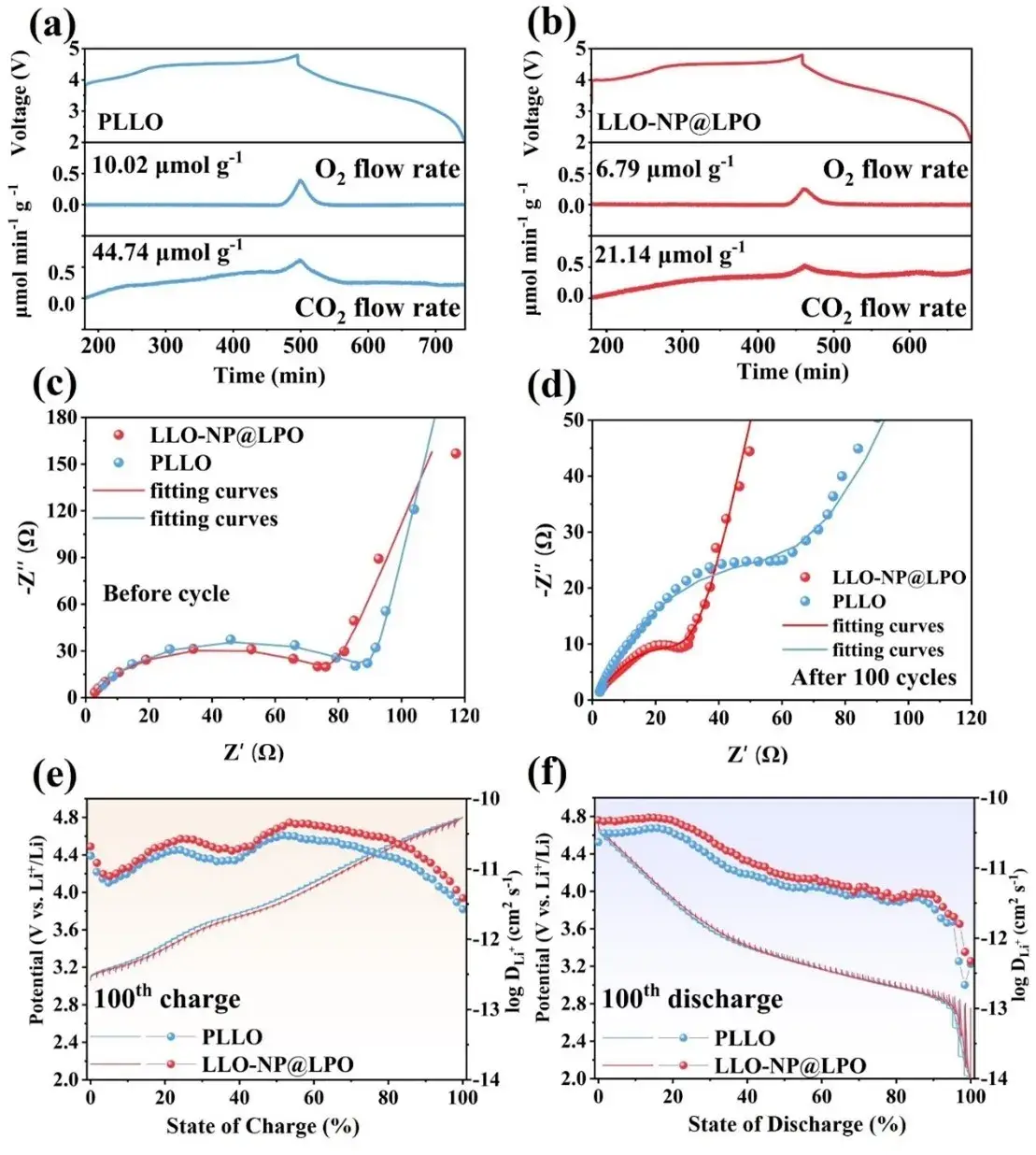
Figure 5. DEMS test during charging and discharging of (a) PLLO and (b) LLO-NP@LPO. Electrochemical impedance spectra of PLLO and LLO-NP@LPO before cycling (c) and after 100 cycling cycles (d). (e, f) GITT curves and corresponding calculated Li-ion diffusion coefficients at 0.2C after 100 cycling cycles.
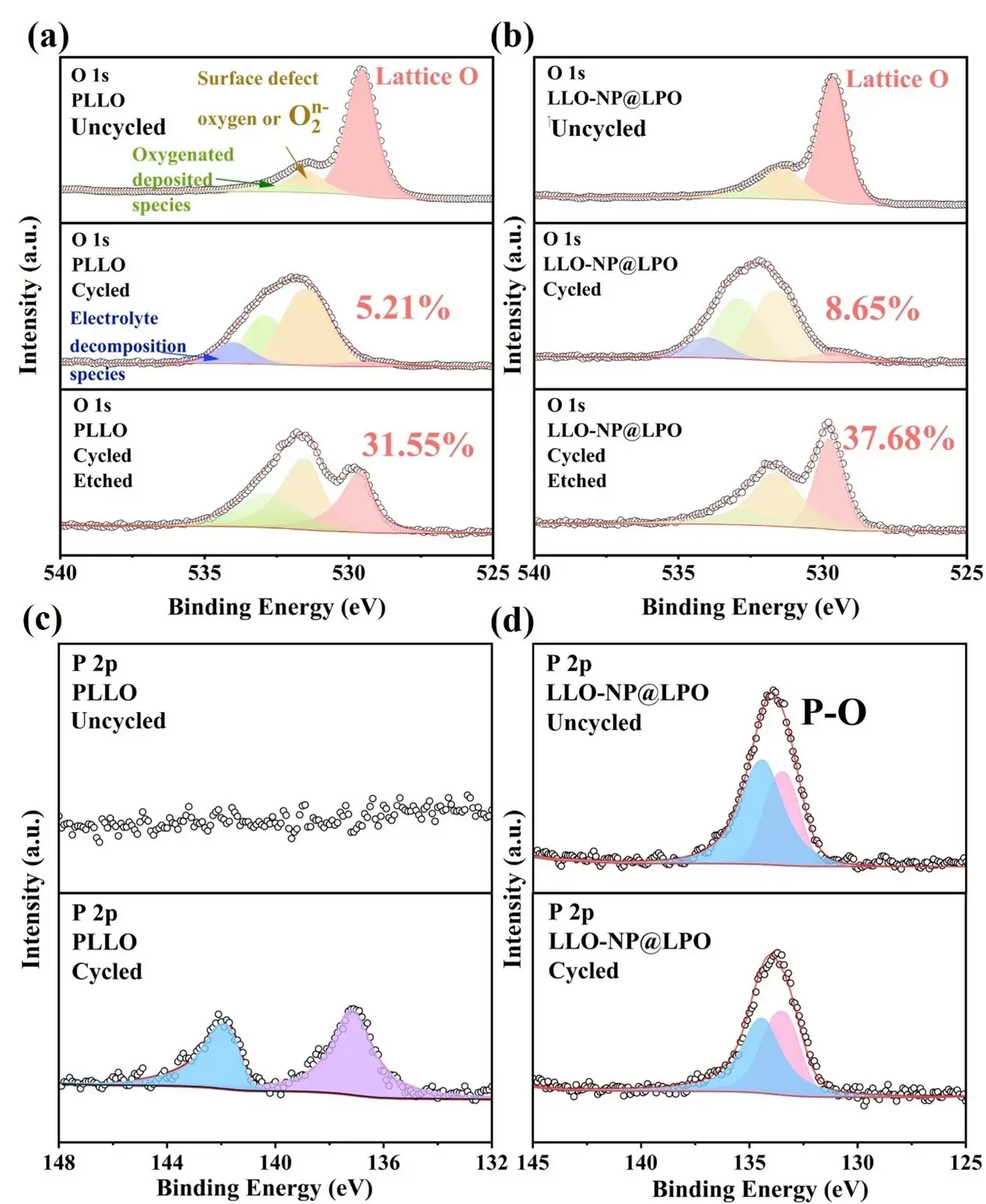
Figure 6. XPS maps of (a, b) O 1s (c, d) P 2p in PLLO and LLO-NP@LPO samples.
7. Mechanical Robustness and Microstructural Preservation
Mechanical testing and microscopy reveal preserved particle integrity:
-
Single-particle compression tests show the average crush force increases from 5.154 mN (PLLO) to 8.143 mN (LLO-NP@LPO), indicating higher mechanical resilience and greater resistance to stress-induced fracture during repeated cycling.
-
After 400 cycles, SEM/TEM show PLLO exhibits particle cracking, internal lattice distortion and partial conversion to spinel/rocksalt phases; by contrast, LLO-NP@LPO retains intact secondary particles, clearer layered lattice fringes and a more uniform CEI.
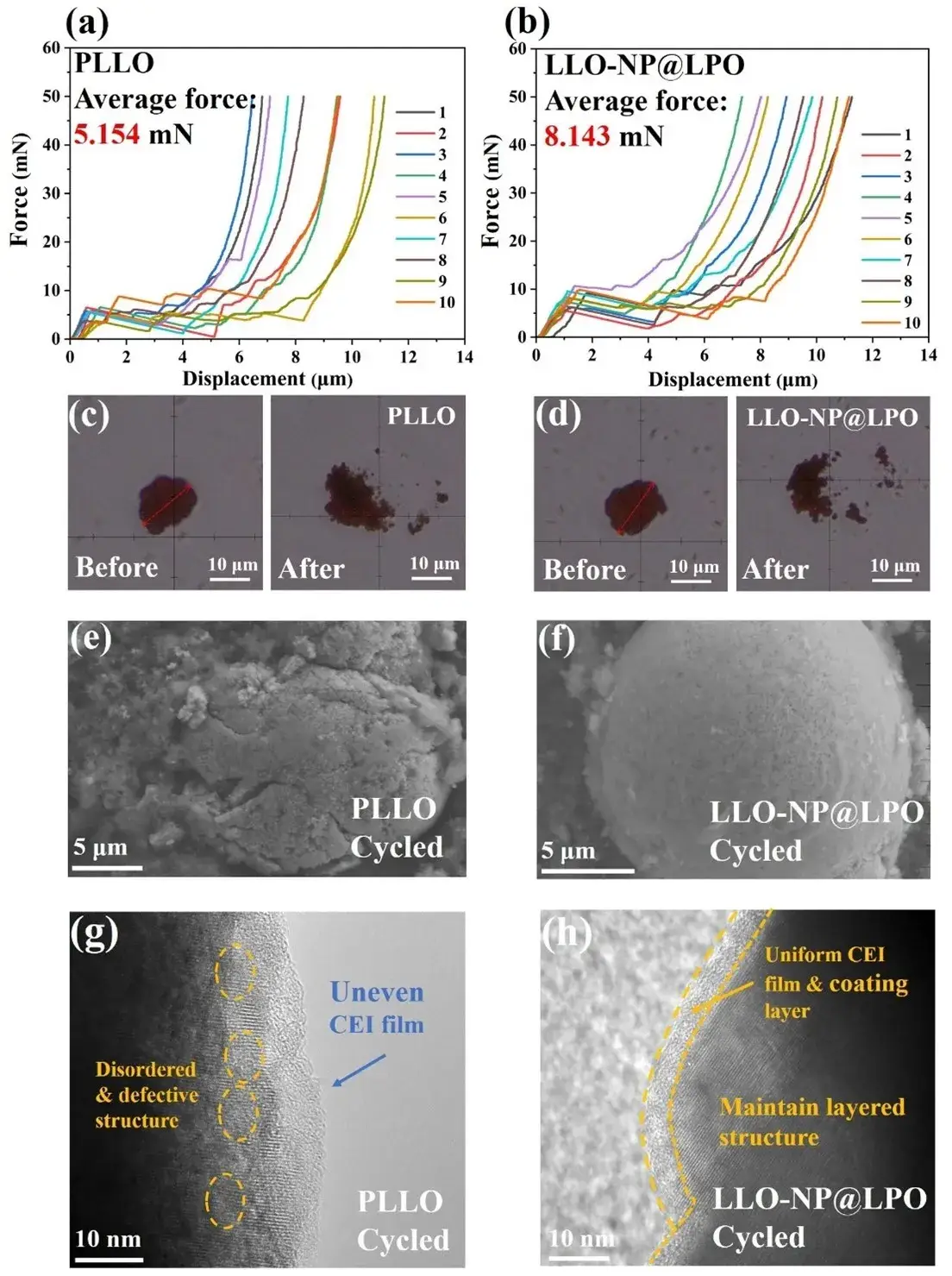
Figure 7. (a) Single particle crushing test curves of PLLO and (b) LLO-NP@LPO. (c, d) Optical photos of PLLO and LLO-NP@LPO before and after single particle crushing test. (e, f) SEM, and (g, h) TEM images of PLLO and LLO-NP@LPO after 400 cycles.
8. Mechanistic Summary: TuningRedox Couples and Stabilizing Lattice Oxygen
In-situ surface reconstruction achieves three synergistic effects:
-
Electronic-structure tuning: Ni²⁺ and PO₄³⁻ lower TM-3d and O-2p energy levels, increasing oxygen redox potential and stabilizing nonbonding O-2p states—this makes oxygen oxidation more reversible and reduces irreversible O₂ release.
-
Interfacial protection: An amorphous Li₃PO₄ coating prevents direct electrolyte attack, suppresses LiPF₆ decomposition products at the surface, and reduces CO₂ evolution.
-
Kinetics and mechanics: Enlarged lattice spacing and a fast-ion surface layer enhance Li⁺ diffusion; improved near-surface composition raises mechanical strength and mitigates stress accumulation and particle collapse.
Together these mechanisms explain the observed gains in capacity retention, voltage stability and high-temperature performance.
9. Summary and outlook
The in-situ near-surface reconstruction strategy—combining Ni²⁺ and PO₄³⁻ near-surface doping with an amorphous Li₃PO₄ coating—provides an effective route to tune redox couples and stabilize lattice oxygen in Li-rich Mn-based cathodes. The approach reduces gas evolution, lowers interfacial impedance, sustains Li⁺ diffusion under high-voltage conditions and strengthens particle mechanics, which together yield substantially improved cycling and thermal stability. Future work should explore optimization of doping depth, PO₄ coverage, and compatibility with practical electrode loadings and electrolytes, and quantify tradeoffs between increased redox potential and long-term electrode conductivity.
10. Original Paper
Zhu Xutao, Xie Xujia, Lin Jie, Liu Yuanyuan, Gao Guiyang, Yang Yong, Zhang Yinggan, Xiong Weicheng, Jiang Yidi, Li Qiyuan, Peng Dongliang*. Tailoring Redox Couples of Li-Rich Mn-Based Cathode Materials by In-Situ Surface Reconstruction for High-Performance Lithium-Ion Batteries. Nano Energy, 2024. DOI: 10.1016/j.nanoen.2024.110588
11. Related Test Equipment Recommendation
You may want to learn about our equipment: IEST Single Particle Mechanical Properties Test System (SPFT2000)
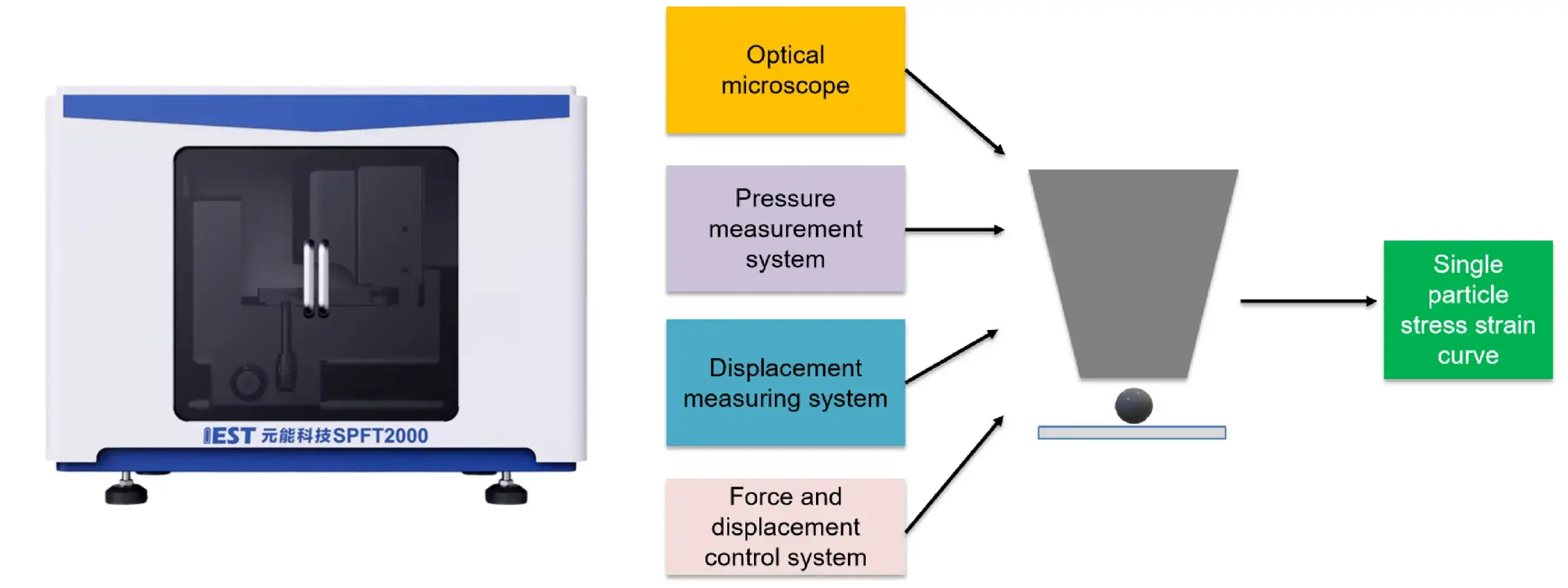
Application:
- Testing the crushing strength of battery material particles
- Can be used to evaluate the pressure resistance of the material
- Guide the rolling process
- Materials with high mechanical strength will have better subsequent cycle stability
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



