-
iestinstrument
Impact of Compaction Pressure On Formation and Performance of Garnet-based Solid-state Lithium Batteries
Journal: Energy Materials
Organization: School of Materials, Xiamen University, IEST Instrument.
Authors: Jie Zhu, Yunfan Wu, Hongyi Zhang, Xujia Xie, Yong Yang, Hongyu Peng, Xiaochun Liang, Qiongqiong Qi, Weibin Lin, Dongliang Peng, Laisen Wang*, Jie Lin*.
Correspondence Authors: WANG Laisen, LIN Jie
Article Link: Impact of compaction pressure on formation and performance of garnet-based solid-state lithium batteries
1. Background
Solid-state lithium battery(SLB) have attracted wide attention due to their high energy density and safety performance, in which garnet-type Li7La3Zr2O12(LLZO)electrolyte possesses high chemical stability and a wide electrochemical window; however, LLZO suffers from low densification, small ionic conductivity, and poor interfacial contact. At present, there have been many researches to improve its performance through elemental doping and high-temperature sintering, but there is a lack of systematic and targeted research on the effect of compaction pressure on the molding and performance of LLZO solid-state lithium battery.
2. Article Highlights
Recently, Prof. Peng Dongliang’s team from the School of Materials of Xiamen University and IEST Instrument company. published a research paper entitled “Impact of Compaction Pressure On Formation and Performance of Garnet-based Solid-state Lithium Batteries” in the journal Energy Materials. Based Solid-State Lithium Batteries”. In the article, Ta-doped LLZO (LLZTO) was used as the research object to prepare solid-state electrolyte by high-temperature solid-phase method, and the impact of compaction pressure on the performance of LLZTO solid-state lithium battery was investigated by changing the compaction pressure before high-temperature sintering. The compaction pressures of 50, 150, 300 and 600 MPa were recorded as LLZTO-50, LLZTO-150, LLZTO-300 and LLZTO-600, respectively, and the results showed that increasing the pressure could improve the crystallinity and make the structure denser, and the ionic/electronic conductivity was also increased, among which the densification and the ionic conductivity were the highest for the LLZTO-600 sample, respectively. ionic conductivity were the highest, 94% and 6.36×10⁻⁴ S cm⁻¹, respectively. The Li|LLZTO-600|Li symmetric battery was able to cycle stably for 1500 h without short-circuit, and the discharge specific capacity of the LFP|LLZTO-600|Li full battery after 150 cycles was 158%. The discharge specific capacity of the LFP|LLZTO-600|Li full cell was 158.4 mAh g⁻¹ after 150 cycles, and the capacity retention rate was as high as 94.8%. This study shows that the selection of appropriate compaction pressure plays a key role in the morphology and electrochemical performance of LLZTO, and provides a reference for the preparation of other high-performance solid-state lithium battery.
3. Results and Discussion
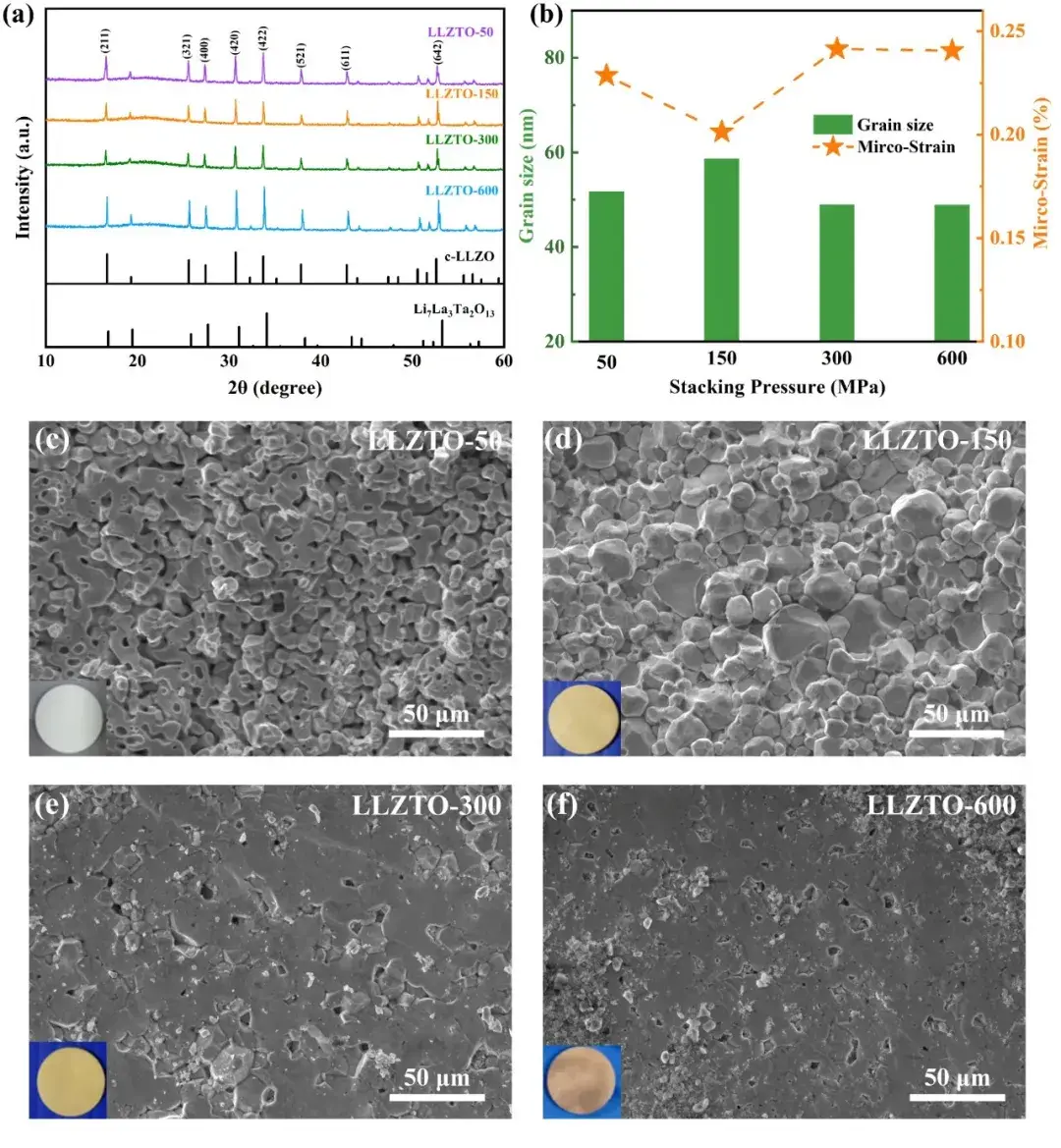
Figure 1. (a) XRD of different LLZTO powders; (b) grain size and microstrain of different LLZTO powders; (c) SEM cross-sections and photomicrographs of LLZTO-50, (d) LLZTO-150, (e) LLZTO-300, and (f) LLZTO-600 solid state electrolyte sheets.
The XRD results showed that the characteristic peaks of each sample were well matched with c-LLZO, and the characteristic peaks and splitting peaks were more pronounced with increasing compaction pressure, indicating that the crystallinity of SSE was improved (Fig. 1a). The calculated grain size and microstrain (Fig. 1b) increased and then decreased with increasing compaction pressure, and the microstrain decreased and then increased, indicating that the larger the compaction pressure, the larger the deformation, the smaller the grains, and the denser the structure.The SEM image showed that the LLZTO-50 grains (Fig. 1c) were not in sufficient contact with each other, and many pores appeared in the worm-like structure.The LLZTO-150 grains ( Fig. 1d) are in contact with each other but not fully dense, showing irregular hexagonal grains.LLZTO-300 particles (Fig. 1e) are in close contact with each other, and most of the grain boundaries have disappeared due to grain fusion, except for a few tiny pores.The close contact between LLZTO-600 particles (Fig. 1f) results in the densest grain morphology, with no grain boundaries and very few pores.
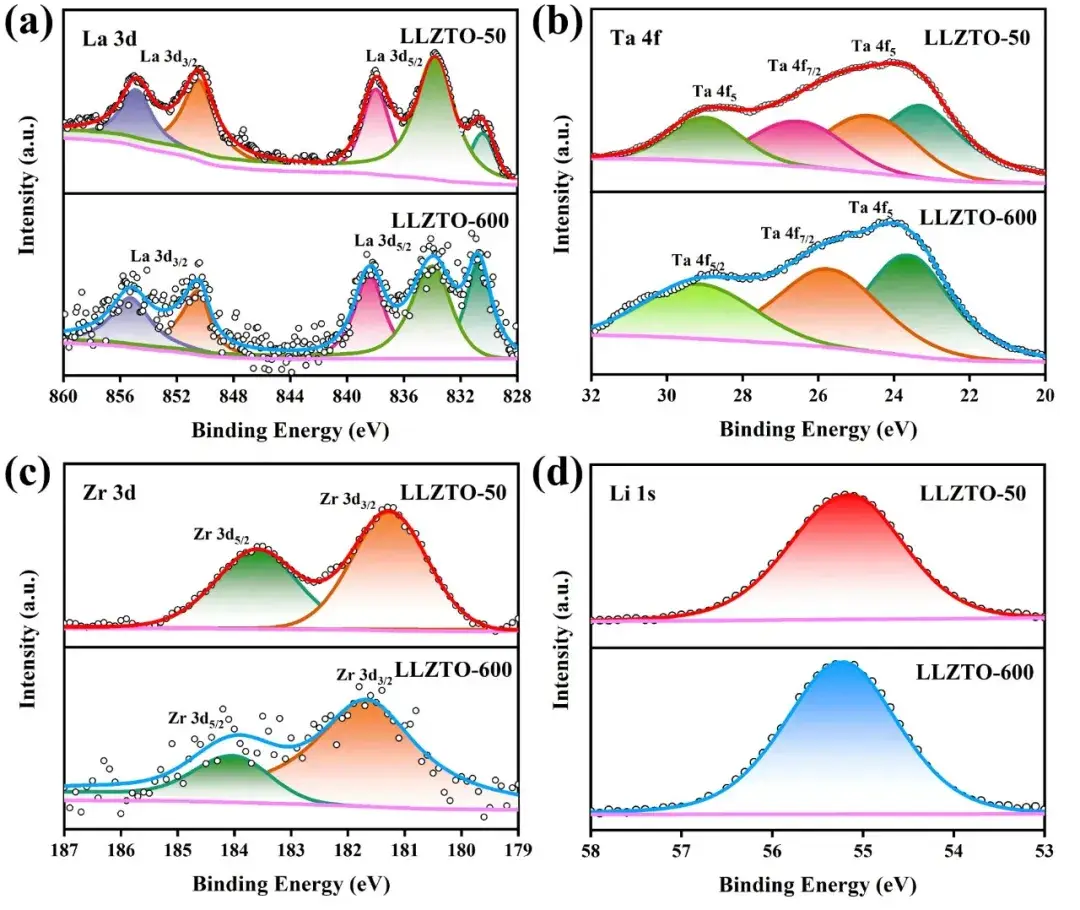
Figure 2. (a) La 3d, (b) Ta 4f, (c) Zr 3d, (d) Li 1s XPS results for LLZTO-50 and LLZTO-600 powders.
The XPS test results indicate that the element La (Fig. 2a) exists mainly in the form of metal-like oxides. The Ta 4f peak of LLZTO-50 consists of split peaks, which corresponds to Ta3+ oxides, whereas the Ta 4f peak of LLZTO-600 is shifted toward higher binding energy, suggesting that Ta is more oxidized in the high densification pressure samples (Fig. 2b).The Zr 3d peak of LLZTO-600 is also shifted toward higher binding energy (Fig. 2c), suggesting that Zr is more highly valent in the high densification pressure samples. peak is also shifted toward higher binding energy (Fig. 2c), indicating higher valence of Zr in the high densification pressure samples. The Li 1s peaks of LLZTO-50 and LLZTO-600 (Fig. 2d) are similar, but the area of the peaks is larger, and the loss of lithium during high-temperature sintering is suppressed. The above results indicate that the high compaction pressure in the samples resulted in higher densification, suppressed lithium loss and increased oxygen concentration, and led to more complete transition metal oxidation.
Table 1. Compactness, ionic conductivities, activation energies and electronic conductivities of different LLZTO samples.
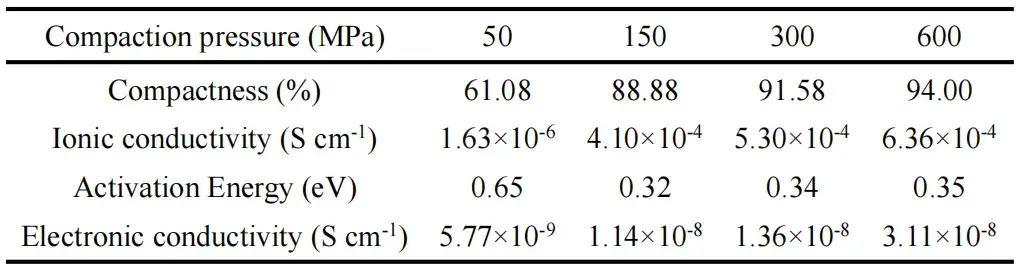
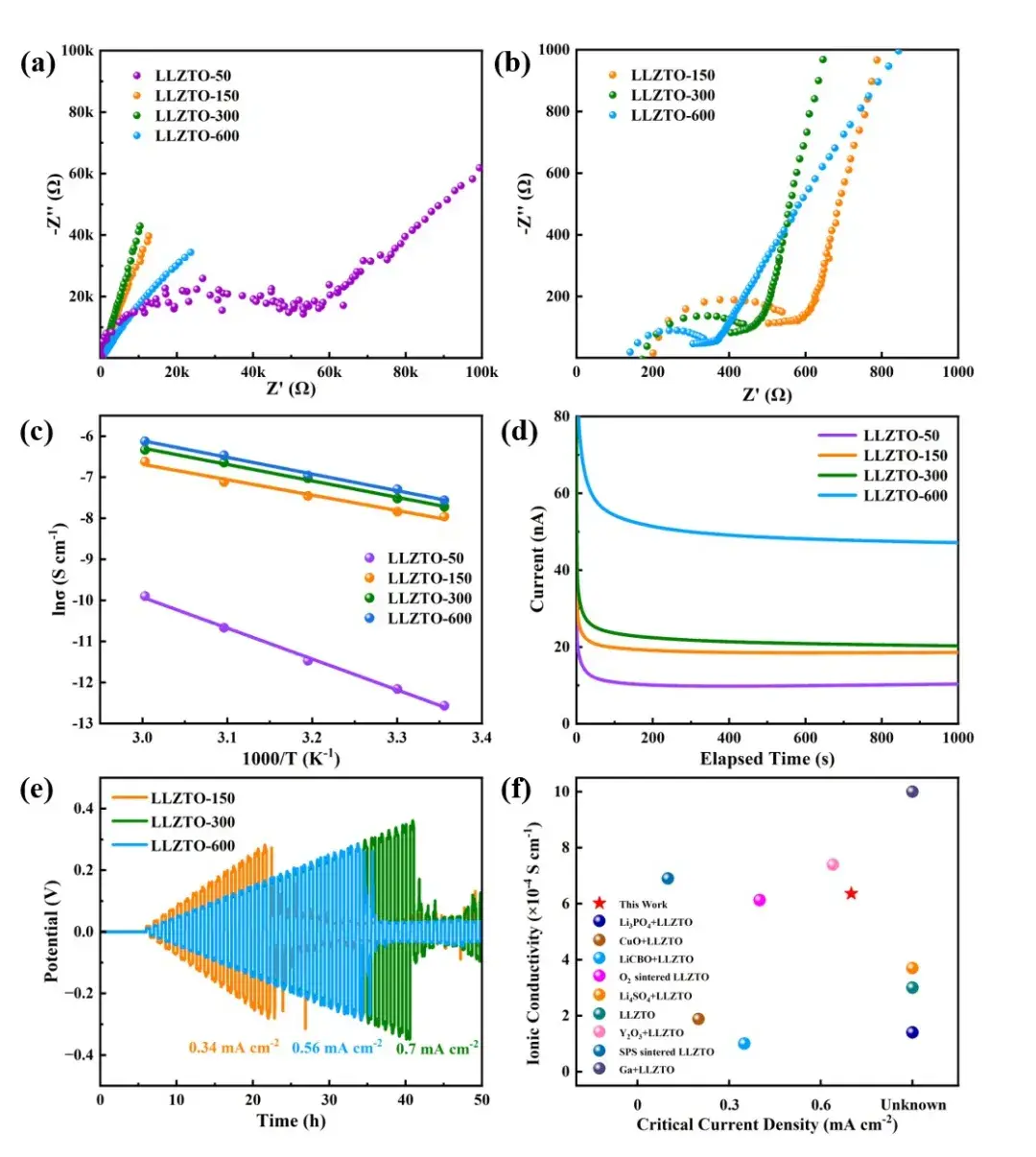
Figure 3. (a) Overall EIS, (b) amplified EIS and (c) Arrhenius curves for different LLZTO samples. (d) DC polarization curves for different Ag|LLZTO|Ag symmetric cells. (e) Critical current density (CCD) for different Li|LLZTO|Li symmetric cells. (f) Performance comparison of the present work with other findings.
The densification of the solid electrolyte sheet increased with increasing compaction pressure (Table 1).The EIS results (Fig. 3a-b) showed that the total resistances of LLZTO-50, LLZTO-150, LLZTO-300, and LLZTO-600 were 53150, 580, 450, and 366 Ω, respectively, which indicated that the ionic conductivity (Table 1) increased with increasing densification pressure. The activation energy of LLZTO-50 is higher than that of other samples due to more pores and poor inter-particle contact (Fig. 3c). The steady-state currents of LLZTO-50, LLZTO-150, LLZTO-300, and LLZTO-600 were 10, 18, 20, and 47 nA, respectively, when the polarization voltage was applied to the Ag|LLZTO|Ag symmetric cell (Fig. 3d), suggesting that the electronic conductivity also increased with the increase of densification pressure. the steady-state currents of LLZTO-150, LLZTO-300, and LLZTO-600 had critical current densities (CCDs) of 0.34, 0.70, and 0.56 mA cm-2 , respectively (Fig. 3e).The high electronic conductivity of LLZTO-600 (Table 1) meant that it could not withstand repetitive lithium deposition with high currents, and so, despite the higher densification pressure of LLZTO-600, it had a lower CCD when compared with LLZTO-300, but the the ionic conductivity and CCD of the LLZTO-600 sample were still superior compared to the reported results (Fig. 3f).
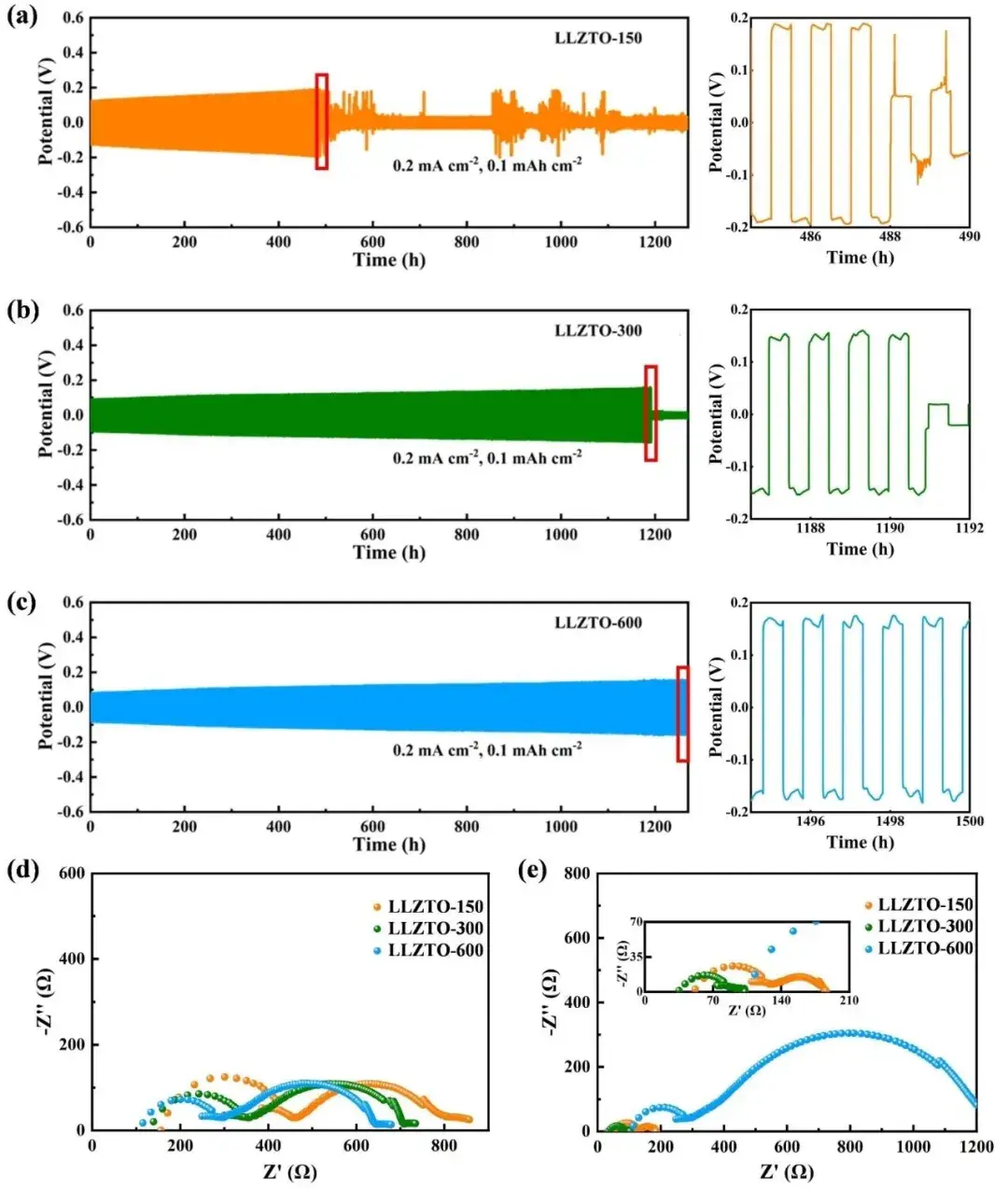
Figure 4. Cycling performance of (a) LLZTO-150, (b) LLZTO-300, and (c) LLZTO-600 lithium-symmetric batteries at the same current density; EIS of lithium-symmetric batteries (d) before cycling and (e) after 1500 cycles.
The stability of the lithium interface was evaluated by symmetric batteries. The initial polarization voltage of Li|LLZTO-150|Li was ~120 mV (Figure 4a), and gradually increased at a rate of ~0.12 mV/cycle, and a short circuit occurred after 487 cycles. The initial polarization voltage of Li|LLZTO-300|Li was ~80 mV (Figure 4b), and the battery cycled at a current density of 0.2 mA cm-2 without obvious fluctuations. In 1190 cycles, the overpotential increase rate was 0.06 mV/cycle, which was only half of that of Li|LLZTO-150|Li, indicating that LLZTO-300 particles have better interfacial stability to lithium metal and inhibit the growth of lithium dendrites. Li|LLZTO-600|Li has the longest cycle life (Figure 4c) because of its highest ionic conductivity and largest material density. The total resistance of the lithium-lithium symmetric batteries of LLZTO-150, LLZTO-300 and LLZTO-600 before cycling (Figure 4d) were 800, 700 and 640 Ω, respectively. After 1500 cycles (Figure 4e), the resistance of the LLZTO-150 and LLZTO-300 lithium symmetric batteries was very small (180 and 99 Ω) due to short circuit, while the total resistance of Li|LLZTO-600|Li increased to 1230 Ω due to interfacial reaction.
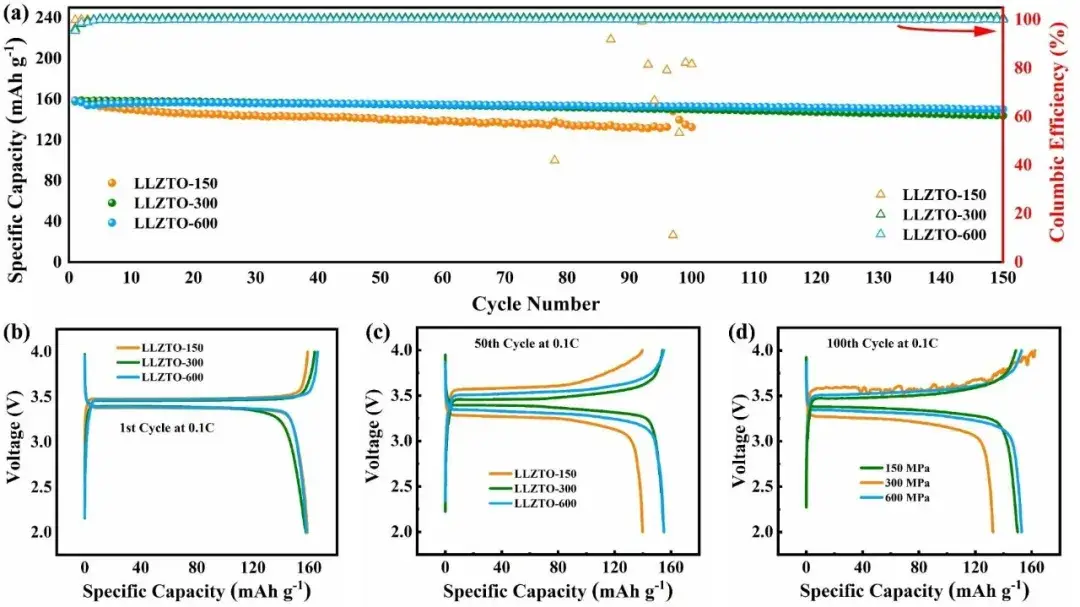
Fig. 5. (a) Cycling performance and Coulombic efficiency of LiFePO4|LLZTO|Li full cell; (b) Charge-discharge curves corresponding to the 1st, 50th, and 100th cycles at 0.1C.
The practical performance of this solid-state electrolyte was evaluated by assembling a full cell with LiFePO4 (LFP) cathode and lithium cathode.The LFP|LLZTO-150|Li (Fig. 5a-b) full cell had an initial discharge capacity of 158.9 mAh g-1 and an initial Coulombic efficiency (CE) of 99.9%, but the capacity decayed severely in subsequent cycles.The LFP|LLZTO- 300|Li has an initial discharge capacity of 157.9 mAh g-1 with an initial CE of 96.3%, and after 150 cycles, the reversible capacity is maintained at 143.8 mAh g-1, with a capacity retention rate of 91.1%.The LFP|LLZTO-600|Li has an initial discharge capacity of 157.9 mAh g-1, with a capacity retention rate after 150 cycles of 94.8%, the best full cell performance. This is due to the high ionic conductivity that facilitates stable lithium ion transport inside the cell and the dense structure that facilitates the stability of the anode/electrolyte/anode interface. At the 50th lap (Fig. 5c), the LFP|LLZTO-150|Li cell exhibits the maximum overpotential, in addition to the sharp voltage fluctuation at the 100th lap (Fig. 5d).The stable cycling and minimum polarization of the LFP|LLZTO-600|Li verifies the feasibility of improving the performance of solid-state lithium battery by adjusting the compactionpressure.
4. Summary and Outlook
In this work, LLZTO solid electrolytes with different densities and properties and their solid-state lithium battery were prepared by adjusting the densification under pressure before high-temperature sintering.The LLZTO-50 sample has a loose structure, while the LLZTO-600 sample has the highest densification (94%) and the highest ionic conductivity (6.36×10-4 S cm-1). Meanwhile, the Li|LLZTO-600|Li symmetric cell showed the best cycling performance, which could be stabilized for 1500 cycles without short-circuiting.The reversible capacity of the LFP|LLZTO-600|Li full cell was 158.4 mAh g-1, and the capacity retention after 150 cycles was as high as 94.8%. This work demonstrates that densification pressure can modulate the component and structural stability of solid-state electrolytes to improve the electrochemical performance of their solid-state lithium battery.
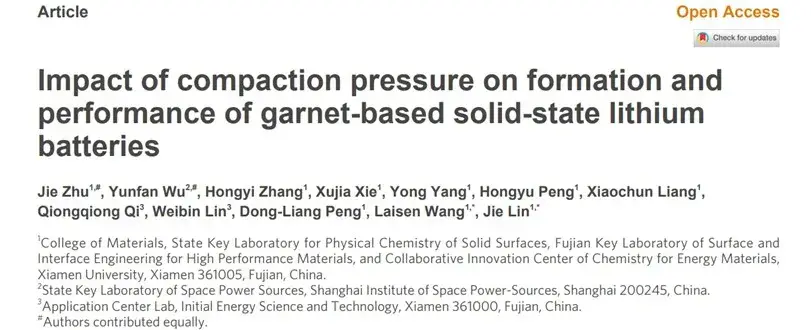
5. Solid-state test system recommendations
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.