-
iestinstrument
Summary of The Latest Breakthroughs In Solid-state Battery
The following are recent developments and innovative approaches in the field of solid-state battery, with some findings excerpted from reports by universities and research institutions.
1. Negative electrode-free all-solid-state battery, latest Nature Energy!
Ying Shirley Meng’s team at the University of California, San Diego, published a paper entitled “Design principles for enabling anode-free sodium all-solid-state battery” in Nature Energy. “This research presents a solution to overcome two major challenges by using an electrochemically stable solid electrolyte and applying stack pressure to achieve dense sodium metal deposition. In addition, it was found that the aluminum collector and the solid electrolyte can achieve tight solid-solid contact for highly reversible sodium plating and stripping at high surface capacity and current density. This design was previously not possible when using conventional aluminum foils. This study demonstrates a sodium-free anode all-solid-state battery that exhibits stable cycling performance after hundreds of cycles.
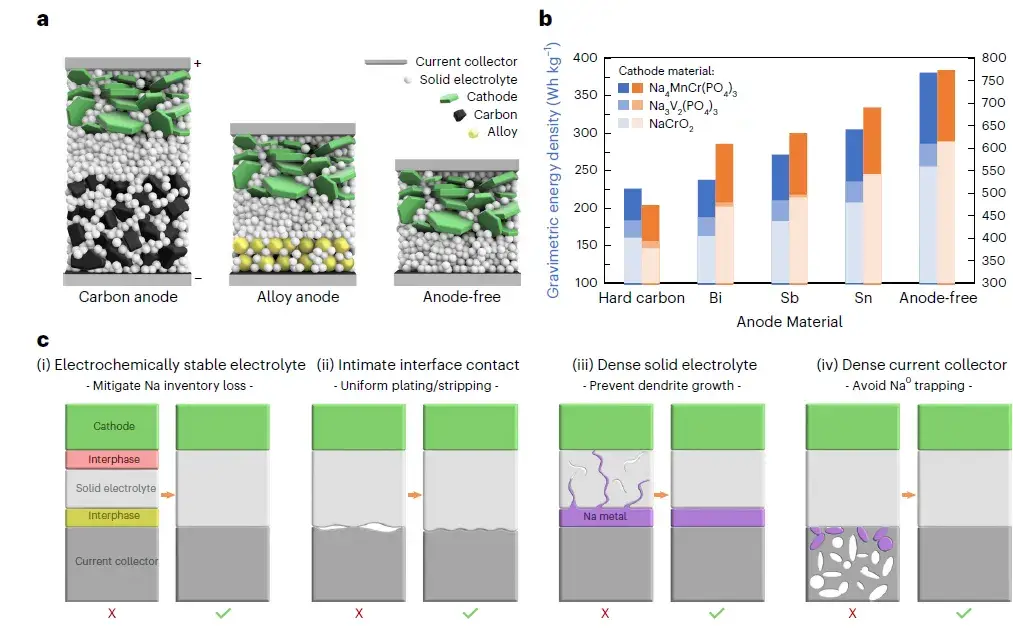
Figure 1. Anode-free schematic and energy density calculations. a, Battery schematic for carbon anode, alloy anode, and anode-free configurations. b, Comparison of theoretical energy densities of various sodium anode materials. c, Schematic diagram of the four requirements for realizing an anode-free all-solid-state battery. d, Schematic diagram of the four requirements for realizing a sodium anode-free all-solid-state battery. © 2024 Springer Nature
2. Adv. Mater.: Highly Safe Lithium Metal Batteries in Solid State by Prof. Jiaqi Huang’s Group at Beijing Institute of Technology
Prof. Jiaqi Huang’s group at the Institute of Frontier Cross-Science, Beijing Institute of Technology (BIT), has made significant progress in the design of high safety lithium metal batteries, and the related results have been published in the journal entitled “Intrinsically Safe Lithium Metal Batteries Enabled by Thermo- electrochemical Compatible In-situ Polymerized Solid-state Electrolytes”. electrochemical Compatible In-situ Polymerized Solid-state Electrolytes” was published in the top international journal of materials, Advanced Materials (Advanced Materials, impact factor 27.4). The corresponding authors of this paper are Prof. Jiaqi Huang and Associate Prof. Hong Yuan of Beijing Institute of Technology (BIT), and the first author is Shijie Yang, a PhD student of Institute of Frontier Cross-Science/School of Materials, BIT.
The solidification of electrolytes has become the ultimate solution to the safety of batteries due to their inherent high thermal stability, immobility and positive/negative electrode compatibility. Self-supported solid-state electrolytes have poor electrode/electrolyte interfacial contact, which is not conducive to the long-term stable cycling of batteries, while in-situ polymerized electrolytes have attracted more and more attention due to better interfacial contact and lower interfacial resistance. However, conventional polyolefin-based polymer electrolytes usually require the addition of an extra liquid electrolyte, which inevitably affects battery safety. In situ polymerized 1,3-dioxolane (PDOL), a polyether-based electrolyte obtained by ring-opening polymerization of DOL, is widely acclaimed not only for its high lithium-ion conductivity (>1 mS cm-1), but also for its excellent compatibility with lithium metal anodes. Although PDOL electrolytes do not require the addition of liquid wetting agents, the presence of DOL monomer residues and oligomers can reduce battery safety. Once the battery temperature exceeds 110 °C, the PDOL electrolyte will thermally decompose and generate gaseous flammable small molecule products (e.g., DOL, formaldehyde, etc.), which will affect the safety performance of the battery. In fact, the thermal safety of lithium metal batteries is mainly related to the thermal stability of the electrolyte and its compatibility with the lithium metal anode at high temperatures. Therefore, simultaneously achieving high thermal stability and high electrochemical stability of PDOL remains a great challenge for safety lithium metal batteries.
The designed functional TGIC crosslinker can be copolymerized with DOL to simultaneously improve the thermal and electrochemical properties of the polymerized electrolyte, which effectively improves the thermal stability of the polymerized electrolyte, and the thermal decomposition of the TPDOL electrolyte is significantly inhibited, and its thermal stability temperature is increased from 86.5 °C to 307 °C compared with that of the PDOL electrolyte (Figure 2).
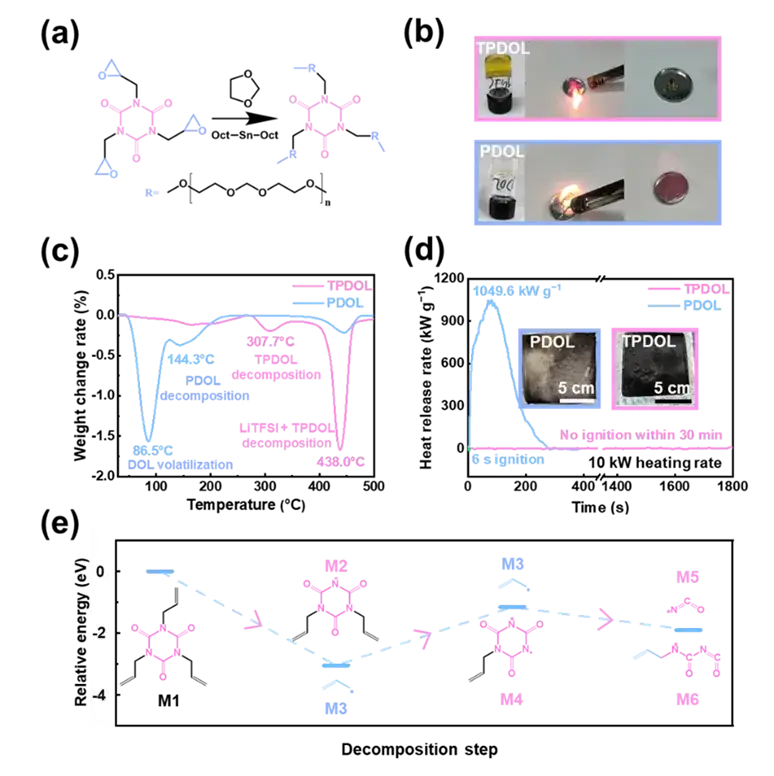
Figure 2. Decomposition step
3. Nat Commun: Long-life creeping all-solid-state battery anode
Considering the unique structural characteristics of solid-state batteries, a group of researcher Suo Lumin at the Institute of Physics, Chinese Academy of Sciences (IPS)/National Research Center for Condensed Matter Physics (NRCP) in Beijing, China, in collaboration with Prof. Xu Rong at the School of Aeronautics and Astronautics, Xi’an Jiaotong University (XJTU), has proposed that lithiation stress can be utilized to drive the creeping of creeping active materials over the cycling cycle, creating a dynamically evolving conformal interface to improve the inter-particle contact and simultaneously dissipate the stresses and improve structural integrity. The authors constructed creeping all-solid-state selenide anodes using creepable Se alloy electrode materials and a rigid Mo6Se8 framework for integrated ion/electron transport, and used in-situ SEM and numerical analyses to thoroughly investigate the lithiation-stress-creep synergistic evolution process and its effect on electrochemical performance. The authors observed that the rigid Mo6Se8 framework can effectively constrain the lithiation strain of Se, generate a lithiation stress gradient, and induce Se creep diffusion into the pore space, which is accompanied by the release of internal stresses and the cumulative formation of conformal interfaces after repeated cycling, which can effectively optimize the particle contact and ultimately lead to the formation of a stable structure under long cycling. Creeping Se-60Mo6Se8 (Se-60MSe) cathode has a volumetric energy density as high as 2460Wh/L, and can be stably cycled for 3000 weeks at 0.5C multiplication rate. Based on the structural properties of solid-state electrodes, this work transforms the interfacial contact from the traditional forced contact delithiation to the active following conformal evolution with cycling from the material intrinsic stress-strain mechanism, which can effectively improve the stability of the electrode structure and provide more insights for the innovative design of future all-solid-state batteries.
The work was published in Nature Communications under the title of “Creep-type all-solid-state cathode achieving long life”. Correspondence should be addressed to Prof. Rong Xu and Prof. Luomin Suo; first author Xiaolin Xiong.
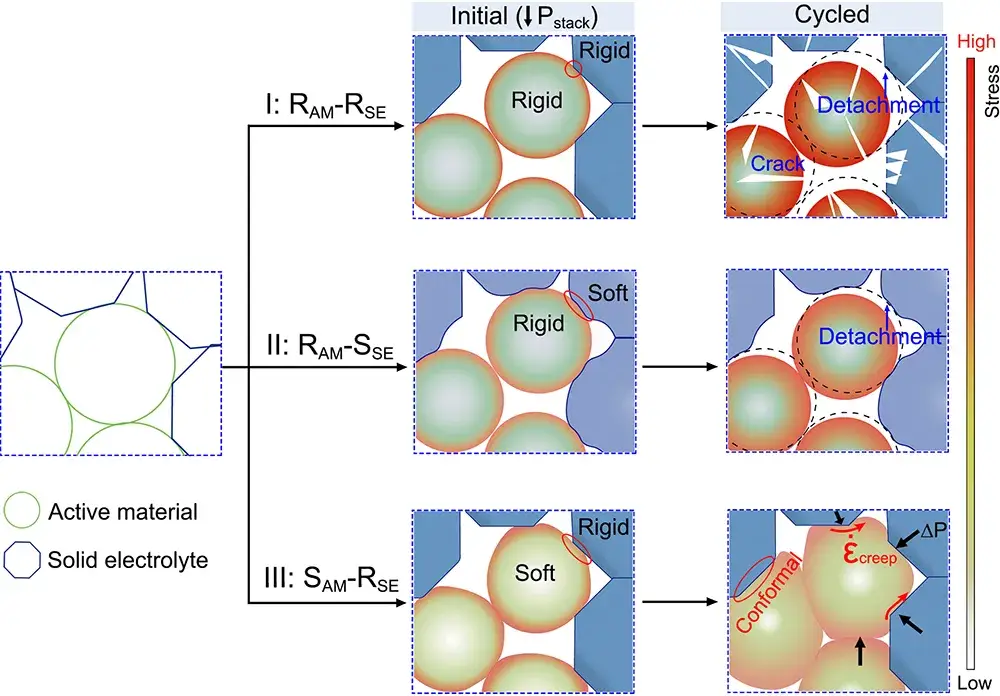
Figure 3. Interfacial evolution inside all-solid-state cathodes.
4. Institute of Metals and others reveal atomic-scale failure mechanism of all-solid-state lithium cathode materials
Chunyang Wang, a researcher in the Department of Material Structure and Defect Research at the Shenyang National Research Center for Materials Science, Institute of Metals, Chinese Academy of Sciences, together with the team of Prof. Yinglin Xin at the University of California, Irvine, USA, have made progress in the study of the failure mechanism of all-solid-state battery cathode materials based on their previous research results on the failure mechanism of liquid lithium cathode materials. The team utilized artificial intelligence-assisted transmission electron microscopy to reveal the atomic-scale structural degradation mechanism of all-solid-state lithium layered oxide cathode materials, and found that the degradation mechanism is significantly different from that in conventional liquid batteries.
It is shown that lattice oxygen loss and surface “lattice fragmentation” driven by local stress coupling and delithiation-induced shear phase transition in all-solid-state batteries jointly lead to the structural degradation of the layered oxides. The surface “lattice fragmentation” involves the formation of nanoscale polycrystalline rock salt phases. This failure mode is found in layered oxide cathode materials. In addition, a new configuration of the shear interface and the formation of large-size O1 phases, which is different from that of the laminated cathode in conventional lithium-ion batteries, were also discovered.
The above results extend the theory of phase transition degradation of layered oxide cathode, which is expected to provide theoretical guidance for the optimized design of cathode materials and cathode/electrolyte interface in all-solid-state batteries.
The research results were published in the Journal of the American Chemical Society (JACS) under the title of Atomic Origin of Chemomechanical Failure of Layered Cathodes in All-Solid-State Batteries.
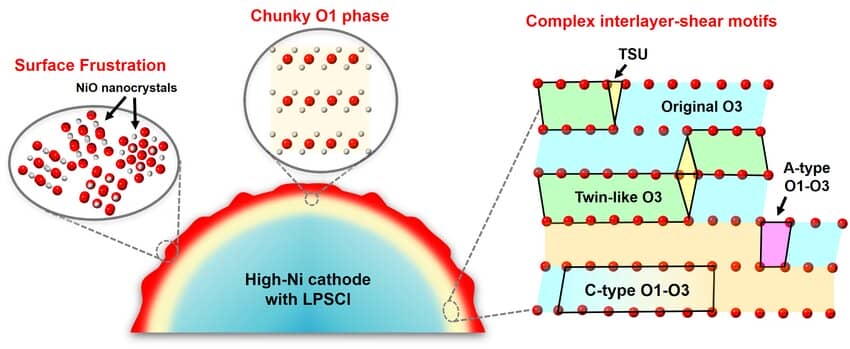
Figure 4. Electrochemically triggered atomic-scale degradation of high-Ni cathodes in ASSBs. The high-Ni cathodes paired with LPSCl degrade through violent surface oxygen loss and nanocrystallization (surface frustration) coupled with subsurface interlayer-shear-induced phase transformation as well as complex interfaces/transition motifs.
5. University of Science and Technology of China (USTC) Successfully Develops Low-Cost Sulfide Solid-State Electrolyte
Prof. Ma Zeng of the University of Science and Technology of China (USTC) has developed a new type of sulfide solid-state electrolyte. While demonstrating the inherent advantages of sulfide solid state electrolytes, this solid state electrolyte achieves an extremely low, commercially viable cost that is unattainable with other sulfide solid state electrolytes. In this study, Prof. Ma took a completely different strategy: instead of working to reduce the cost of lithium sulfide, he developed a sulfide solid-state electrolyte that does not use lithium sulfide as a raw material – lithium phosphorus oxy-sulfide. This solid electrolyte can be synthesized from hydrated lithium hydroxide and phosphorus sulfide as raw materials. As both substances are low-cost, the raw material cost of lithium phosphorus sulfide is only 14.42 U.S. dollars per kilogram, which is less than 8% of other sulfide solid-state electrolytes, and much lower than 50 U.S. dollars per kilogram of the commercialization requirements, which is very cost-competitive.
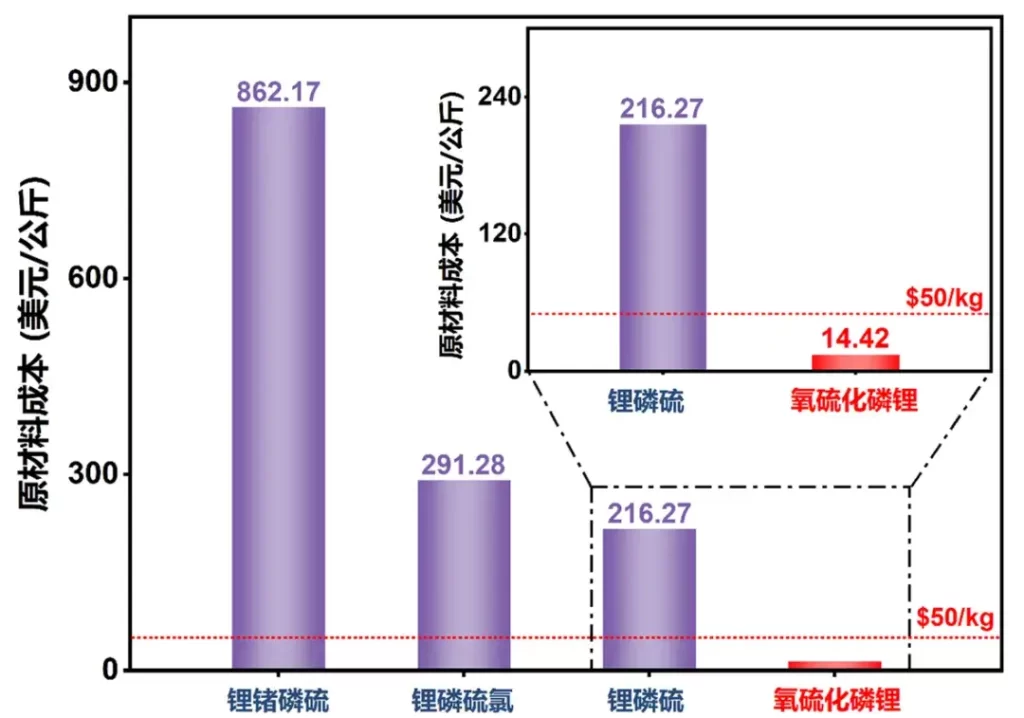
Figure4. Comparison of raw material costs between lithium phosphorus oxy-sulfide and other sulfide solid electrolytes
At the same time, lithium phosphorus oxide also inherits the unique advantage that distinguishes sulfide solid state electrolytes from oxide and halide solid state electrolytes: it has both extremely low density and good anode compatibility. With a density of only 1.7 grams per cubic centimeter (g/cm3), lithium phosphorus oxide has a density similar to that of other sulfide solid state electrolytes, and is lower than that of halide (about 2.5 g/cm3) and oxide solid state electrolytes (mostly higher than 5 g/cm3). At the same time, lithium phosphorus oxy-sulfide shows good compatibility with lithium metal and silicon, two kinds of high-energy-density anode; the symmetric battery composed of it and lithium metal can achieve more than 4,200 hours of room-temperature stable cycling, and the all-solid-state pack battery composed of it, silicon anode and high-nickel ternary cathode still has 89.29% capacity retention rate after 200 cycles at 60 degrees Celsius. The results were published as “A Cost-Effective Sulfide Solid Electrolyte Li7P3S7.5O3.5 with Low Density and Excellent Anode Compatibility” in the international journal Angewandte. Angewandte Chemie International Edition.
6. IEST Solid Electrolyte Test System(SEMS1100) Recommended

IEST is a high-tech enterprise that focusing on R&D and production of lithium battery testing equipments, a professional manufacturer that integrating laboratory instrument R&D and production, method development, instrument sales and technical services. Committed to providing leading testing solutions and services for the global new energy field.
SEMS1100: A multi-functional testing system dedicated to solid electrolyte samples, a fully automatic measurement equipment for the electrochemical properties of solid electrolytes that integrates tableting, testing and calculation. The system adopts an integrated design, including a pressurization module, an electrochemical test module, a density measurement module, a ceramic sheet pressing and clamping module, etc. This equipment can be used for electrochemical stability window, powder tablets, ionic conductivity, electronic conductivity, solid-state battery cycle performance.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



