-
iestinstrument
How Coating Modifies Separator Ionic Conductivity in Li-ion Batteries
1. Abstract
Separator performance directly shapes lithium-ion battery behaviour. In addition to electrical insulation, a separator must enable rapid Li⁺ transport, retain electrolyte, resist heat shrinkage, and survive mechanical handling. Coating a microporous polyolefin support (PE/PP) with polymeric or ceramic layers improves wettability, thermal stability and mechanical strength. However, coatings also change porosity, tortuosity and effective thickness — all of which affect ionic conductivity through the separator. This article summarizes a controlled EIS-based measurement workflow, experimental results on four membranes (base film A and coated membranes B–D), and practical design insights for separator layer coating that preserve or improve ionic conductivity while delivering safety benefits.
2. Preface
The separator is a critical component in lithium-ion batteries, directly influencing key performance metrics such as current output, capacity, and cycle life [1]. An ideal separator must possess several characteristics: (1) excellent electrolyte wettability and ion permeability; (2) superior thermal stability to prevent short circuits from thermal shrinkage; (3) electronic insulation and electrochemical stability; (4) chemical inertness towards electrodes and electrolyte; (5) high mechanical strength to withstand manufacturing stresses; and (6) appropriate thickness, pore size, and porosity to optimize ion transport while balancing mechanical integrity and internal resistance.
Microporous polyolefin separators, notably polyethylene (PE) and polypropylene (PP), dominate the commercial market due to their favorable mechanical properties, electrochemical stability, thermal resistance, and low cost. Despite their widespread use, these separators have limitations, including a tendency for thermal shrinkage at elevated temperatures, low intrinsic porosity, and poor electrolyte affinity, which can significantly impair battery performance. Common modification strategies to address these issues include graft modification, coating, and developing new materials or processing techniques [2].
Coating modification involves applying a layer of organic polymer or inorganic ceramic particles onto a polyolefin or alternative base separator. This technique primarily serves three functions: 1) enhancing electrolyte absorption and retention to extend cycle life; 2) improving high-temperature resistance, flame retardancy, and mechanical properties; and 3) potentially adding shutdown functionality for improved safety [3]. While coatings can significantly boost mechanical and thermal performance, maintaining adequate porosity is crucial to ensure unimpeded ion conduction. Verifying that the coated separator meets performance expectations is essential. This study evaluates the impact of different coating processes on ionic conductivity by comparing the base membrane with various coated separators.
3. Experimental Methods
3.1 Testing Equipment
The self-developed multi-channel ionic conductivity testing system (EIC1400M) by IEST, as shown in Figure 1, was used. This equipment includes four battery assembly fixtures (Figure 1(b)) and can perform rapid four-channel electrochemical impedance spectroscopy tests. The pressure range is 0-20 Kg, and the frequency range is 100 KHz to 0.01 Hz.
Figure 1. (a) Multi-channel Ionic Conductivity Testing System: Equipment Overview; (b) Battery Assembly Fixtures
3.2 Test Samples
The samples tested included Base Separator A and three coated separators (B, C, and D) produced using different coating processes.
3.3 Test Procedure & Separator Ionic Conductivity Calculation Method
In the glove box, place the corresponding number of separators into the fixtures and add electrolyte. Place the assembled fixtures into the equipment and apply a 5 kg force. Initiate the experiment via software and after the set dwell time, automatically test the electrochemical impedance spectroscopy (EIS) of the four channels. The testing frequency ranges from 100,000 to 100 Hz. Perform assembly and testing for separators with 1 to 4 layers respectively, obtaining corresponding EIS curves. Fit these curves to establish a baseline, with the intersection of the fitting line and the X-axis representing the impedance Rs for the n-layer separator, as shown in Figure 2(a). Plotting the number of layers on the X-axis and the impedance value per layer on the Y-axis, perform linear regression on the data to obtain the slope, which represents the ionic impedance R of a single-layer separator, as depicted in Figure 2(b).
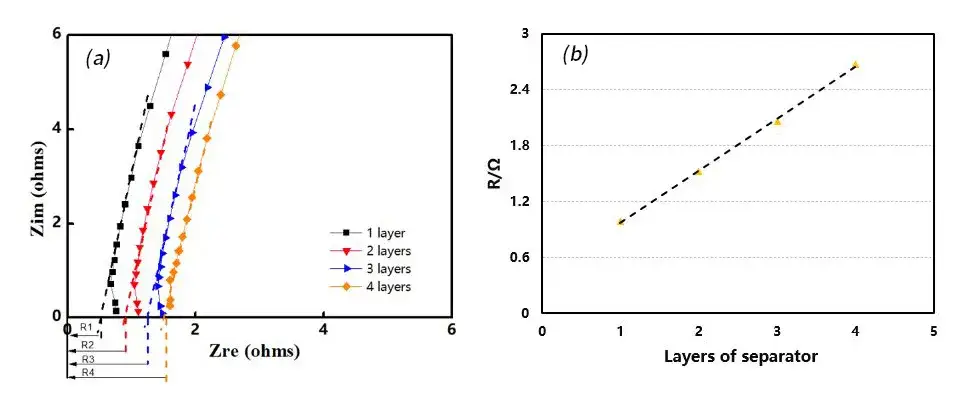
Figure 2. EIS Impedance Spectra of Different Separator Layer Numbers (a); R-Value Fitting Graph (b)
Substituting the obtained ionic impedance R into Formula 1 allows calculation of the separator ionic conductivity.
σ=d /( R * S)(1)
Where σ is the ionic conductivity, d is the thickness of the separator, R is the ionic resistance, and S is the effective surface area of the separator.
4. Results and Discussion
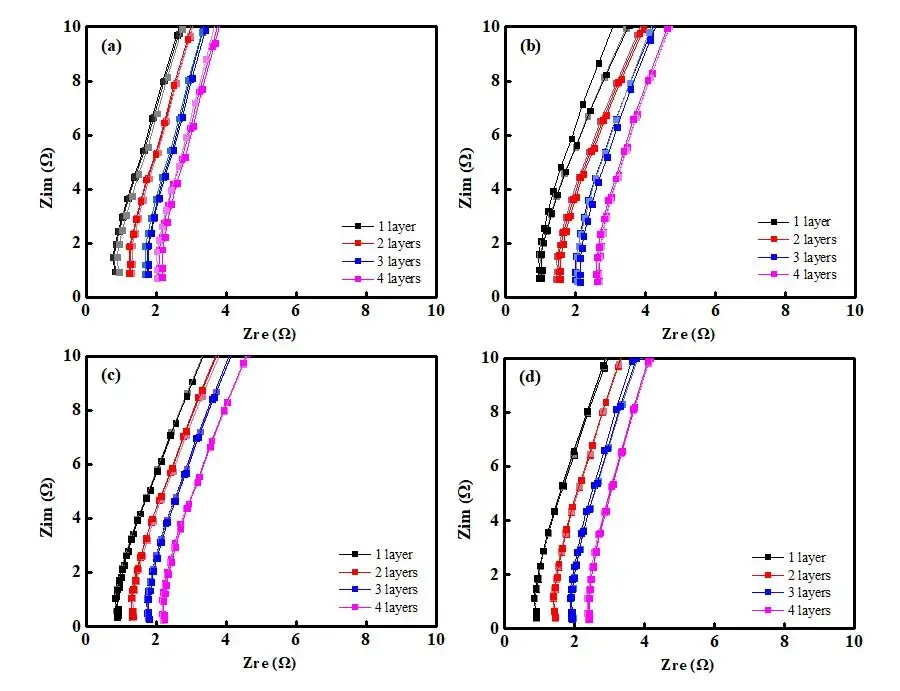
Figure 3. EIS Spectra of Separators with Different Coating Processes: Base Film A (a); Coated Separator B (b); Coated Separator C (c); Coated Separator D (d)
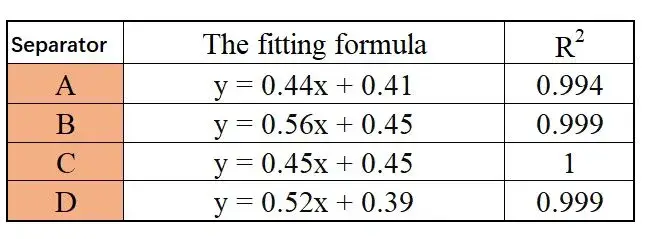
Figure 3 presents the EIS spectra for the different separators. As expected, the impedance increases with the number of separator layers for all samples. The fitted Rs values for 1 to 4 layers are summarized in Table 1. The linear regression plots of layer count versus resistance (Figure 4) and the corresponding equations and goodness-of-fit (R²) are provided in Table 2. The calculated single-layer resistances (R) for separators A, B, C, and D:
-
R (Ω): A = 0.44, B = 0.56, C = 0.45, D = 0.52.
However, using Equation 1 to compute ionic conductivity reveals a more informative picture, the resulting ionic conductivities are:
-
Ionic conductivity σ (S/cm): Separator C (0.00189) > Separator B (0.00169) > Separator D (0.00145) > Separator A (0.00112).
Notably, all coated separators exhibited significantly higher ionic conductivity than the base separator, with improvements of 50.9%, 68.7%, and 29.4% for B, C, and D, respectively, and Separator C showing the largest gain (0.00189 S/cm). This indicates that coating modification provides additional pathways for lithium-ion transport, facilitating improved internal battery ion transport.
Table 1. Fitted Impedance Values and Ionic Conductivity Values of Separators with Different Numbers of Layers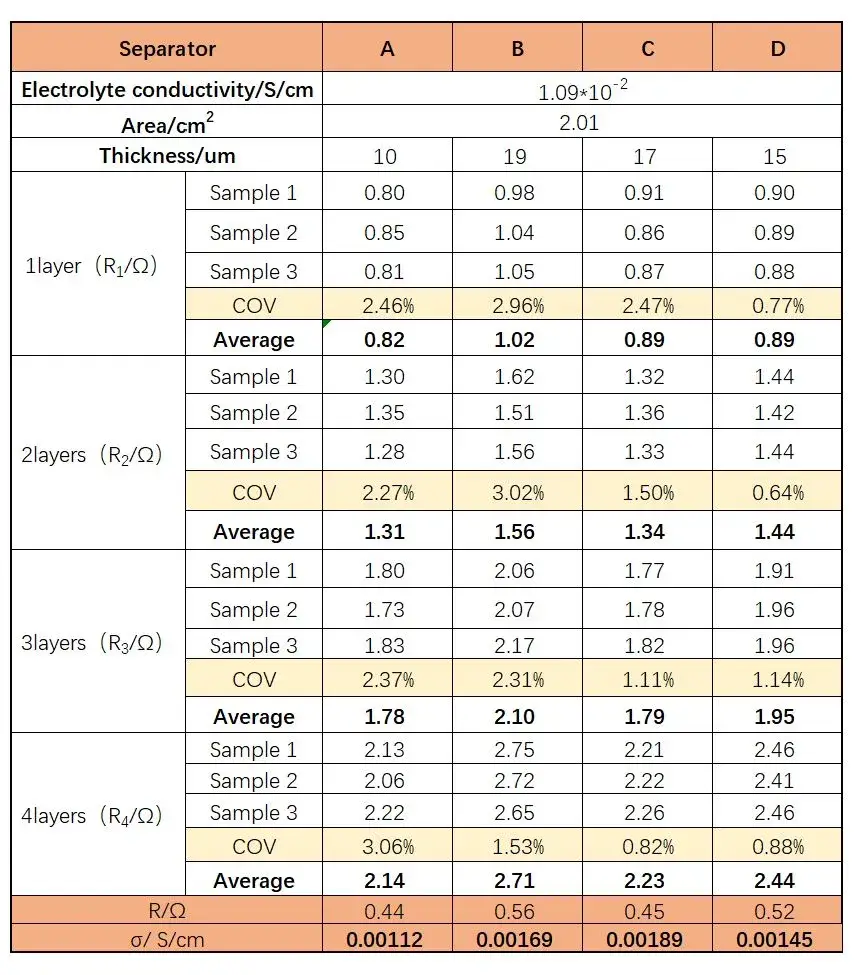
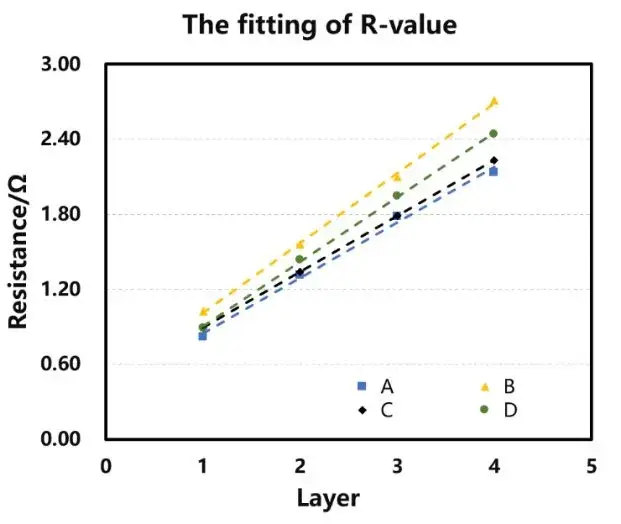
Figure 4. Linear regression graphs of different separators
Table 2. Linear Regression Equations and Coefficients of Determination for Different Separators
5. Interpreting the Coating Influence on Separator Ionic Conductivity
Ionic conductivity is intrinsically linked to the separator’s microstructure, including parameters like pore size, porosity (ε), and tortuosity (τ). Porosity represents the void volume fraction; uniform, moderate porosity helps prevent electrode polarization and lithium plating. Excessively high porosity can compromise mechanical strength and thermal stability, while overly low porosity reduces electrolyte retention and prolongs ion migration paths. Tortuosity, the ratio of the actual ion path length to the separator thickness, directly influences internal resistance. Low tortuosity enables fast ion transport, whereas high tortuosity increases resistance and can promote lithium dendrite growth.
A coating modifies this microstructure. While it increases overall thickness, it can also alter pore structure, size distribution, and interconnectivity. Furthermore, coatings often enhance wettability and electrolyte retention. Therefore, a well-designed coating can create a more favorable micro-environment—with optimized porosity, reduced tortuosity, and better electrolyte access—that more than compensates for the increased physical thickness, resulting in a net gain in ionic conductivity.
6. Practical Guidance for Separator Layer Coating Design
To optimize separator layer coating for ionic conductivity and safety, apply these principles:
-
Prioritize hydrophilic/ion-friendly additives (e.g., ceramic nanoparticles + binder blends) to boost electrolyte uptake without sealing pores.
-
Control coating porosity — use templating or low-viscosity slurries that form interconnected pore networks after drying.
-
Limit effective added thickness: target a coating thickness that meets thermal/mechanical goals while keeping the conduction path short.
-
Minimize tortuosity by aligning pores or using particulate blends that produce straight-through channels.
-
Measure under representative stack pressure — cell compression affects pore geometry; validate σ at mechanical loads similar to cell assembly (the referenced tests used ~5 kgf).
-
Balance trade-offs — increased mechanical/thermal robustness often comes at the cost of added material; quantify the net effect on σ and cell impedance early in development.
7. Conclusion
Coatings can significantly alter separator ionic conductivity through changes to porosity, tortuosity, wettability and thickness. This study effectively employed IEST multi-channel separator ionic conductivity test system to evaluate the performance of separators with different coating treatments, provides a robust, repeatable way to quantify single-layer ionic resistance and to compare coating processes.
Measuring a separator’s ionic conductivity provides direct insight into how easily lithium ions can migrate through it, serving as a critical check on whether a separator, coated or uncoated, meets design expectations. This analytical approach is not only valuable for optimizing coating recipes but also for broader studies investigating the influence of different electrolytes and base separator materials on overall ion transport efficiency.
8. References
[1] Huang Xuejie. Progress in lithium-ion batteries and related materials [J]. China Materials Progress, 2010, 29 (8): 46-52.
[2] Li Jiaxing, Li Feng. Research progress in surface modification technology of polyolefin lithium battery separators [J]. Information Recording Materials, 2021, 22 (4): 3-8.
[3] CHOI J A, SA H K, KIM D W. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators [J], Journal of Power Sources, 2010 (195): 6192-6196.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.