Poor. Frequent disassembly and reconnection lead to inconsistent contact resistance and clamping force.
Electrochemical Impedance Spectroscopy (EIS) is a core electrochemical characterization technique that can non-destructively analyze complex kinetic processes inside batteries, such as lithium-ion migration, charge transfer, and solid-phase diffusion. Key parameters obtained by fitting battery EIS data, like charge transfer resistance, SEI film resistance, and diffusion coefficient, are irreplaceable for accurately assessing battery state of health (SOH), cycle life, failure mechanisms, and performance. Therefore, the accuracy of EIS data directly determines the reliability of subsequent battery state diagnosis and model construction.
Currently, mainstream EIS testing methods are primarily divided into ex-situ and in-situ. Ex-situ testing involves removing the battery from the test equipment and measuring it with a potentiostat under specific conditions, which can easily introduce additional errors due to battery state relaxation (e.g., open-circuit voltage drift, temperature changes). In contrast, in-situ testing involves real-time monitoring during ongoing charge-discharge cycles or rest periods, more accurately reflecting the dynamic impedance information of the battery under actual operating conditions, yielding more authentic EIS data. This article compares EIS data obtained from both in-situ and ex-situ testing methods, analyzes the differences in characterizing battery electrochemical behavior, and thereby argues which method offers superior reliability in ensuring data authenticity and consistency.
In-situ EIS is characterized by an integrated testing architecture, which combines the charge-discharge module and the EIS module on a unified platform, sharing the same signal acquisition system and control unit. This structure ensures consistent baseline current/voltage measurements and eliminates system errors between devices. Impedance measurements are directly conducted during the charge-discharge process, achieving true “online” monitoring.
In this experiment, a 100mAh pouch cell was tested using IEST ERT high-precision battery testing system (as shown in Figure 1a), which integrates the functions of charge-discharge and electrochemical workstations for testing. The test involved charging the battery at 0.2C for 10 minutes, followed by 10 minutes of rest, then discharging at 0.2C for 10 minutes, another 10 minutes of rest, and EIS testing, repeated for 5 cycles as illustrated in Figure 1b. Since the battery had good performance and used low current for short charging and discharging durations, the EIS data from the five cycles are expected to be consistent.
Figure 1. In-situ EIS test equipment (a) ERT Series (b) Test procedure
The core issue of ex-situ EIS testing lies in the discrete and discontinuous nature of the process (Figure 2). Specifically, this method requires researchers to manually transfer the battery from the charge-discharge device to a dedicated EIS testing platform after completing a specific test. This process is cumbersome and prone to contact resistance errors and battery state errors. The experiment used a 100mAh soft-pack battery in a temperature-controlled box, following the same procedure as in in-situ EIS, but after discharging and charging, the battery was removed from the test equipment and placed on the electrochemical workstation for EIS testing, repeating the process for five cycles.
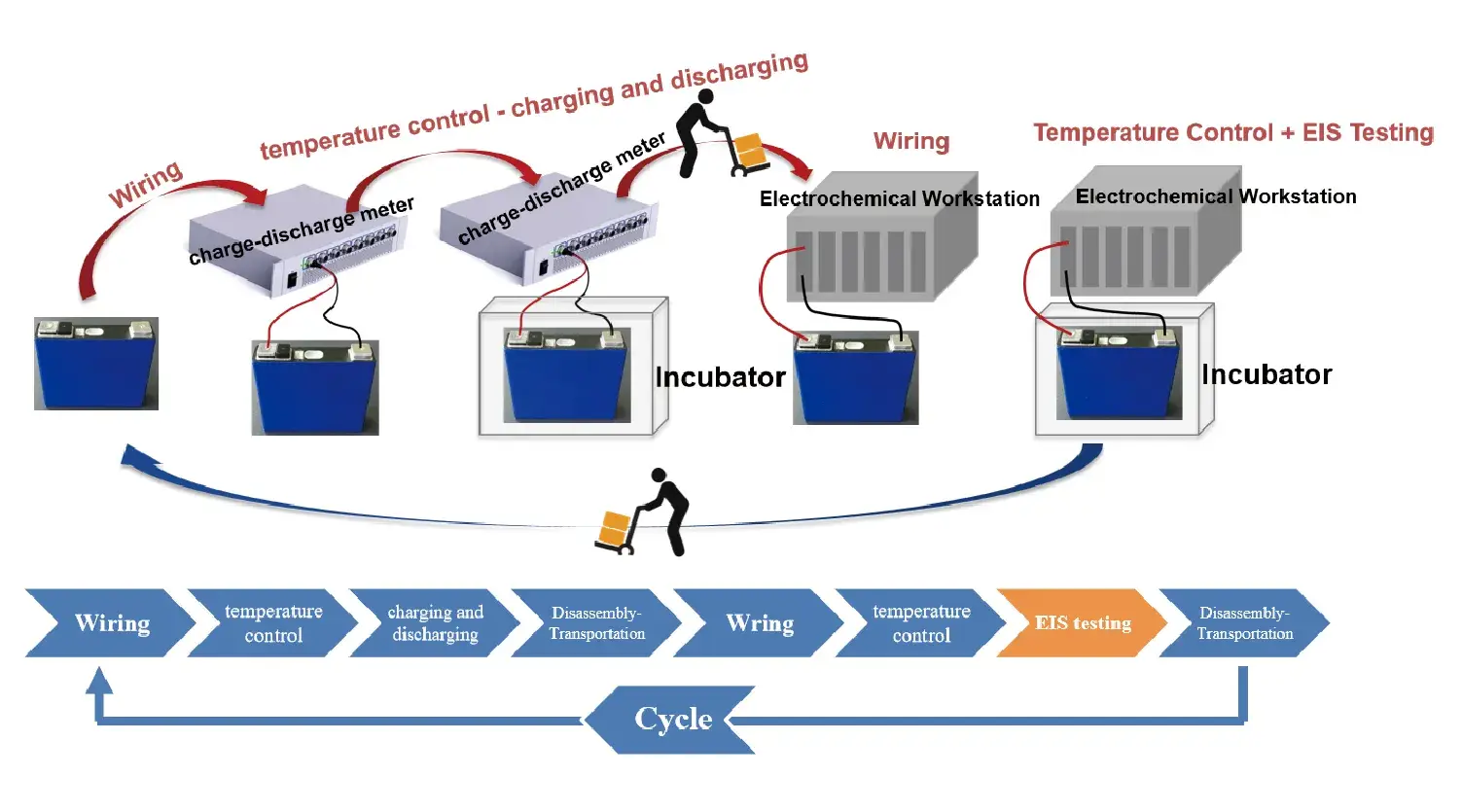
Figure 2. Ex-situ EIS testing process
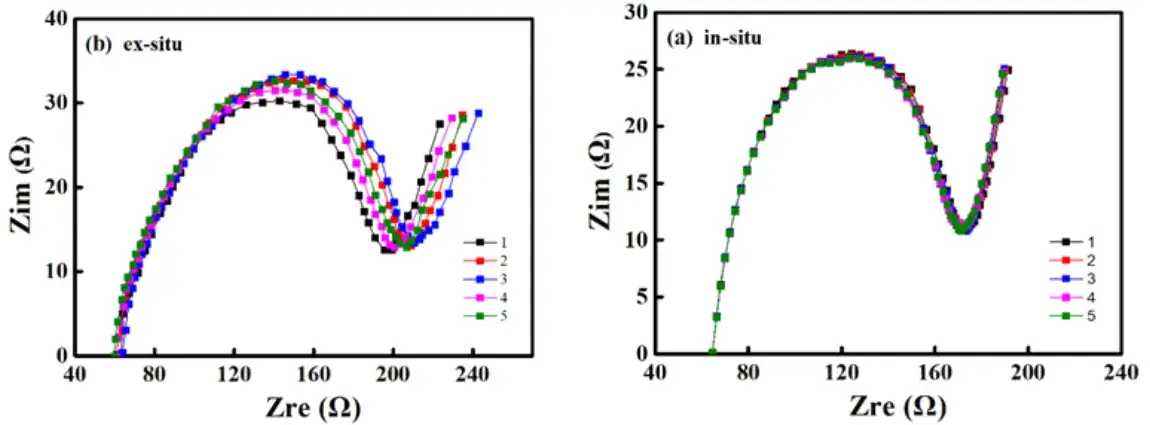
Figure 3. Cyclic EIS spectra: (a) In-situ testing (b) Ex-situ testing
By performing in-situ and ex-situ EIS tests on the same battery model type, analysis of the cyclic EIS data revealed that the EIS curves obtained from in-situ testing were highly overlapping. The fitted Ohmic resistance (Rs) and charge transfer resistance (Rct) values showed minimal fluctuation, with cov ≤1%, demonstrating excellent repeatability. In contrast, the EIS curves from ex-situ testing showed significant dispersion, with variations in Rs and Rct values. This indicates that frequent battery disassembly and reassembly lead to inconsistent contact resistance, and battery state relaxation introduces systematic errors. The experimental results show that in-situ EIS testing, by avoiding manual operation interference, can provide more reliable and accurate impedance data, enhancing data comparability and analytical value.
Table 1: Rs and Rct Fitted from In-Situ and Ex-Situ EIS
|
Test Method |
Number of Cycles |
Rs/mΩ |
Rct/mΩ |
|
In-situ EIS |
1 |
64.6 | 107.5 |
|
2 |
64.5 |
109.3 |
|
|
3 |
64.5 | 108.8 | |
|
4 |
64.5 | 109.3 | |
| 5 | 64.4 |
106.8 |
|
| cov/% | 0.1% |
1.0% |
|
|
Ex-situ EIS |
1 |
60.8 | 138.5 |
|
2 |
62.9 | 146.0 | |
| 3 | 63.7 |
146.7 |
|
| 4 | 61.5 |
139.1 |
|
|
5 |
60.2 |
146.5 |
|
| cov/% | 2.3% |
2.9% |
Based on the test results of in-situ and ex-situ EIS, we can compare the differences between the two methods, as shown in the table below:
Table 2. Comparison of differences between in-situ and ex-situ EIS
| Dimension | In-Situ EIS | Ex-situ EIS | Impact on Mechanism Analysis |
| Connection Consistency | Excellent. Single connection; mechanical connection state |
Poor. Frequent disassembly and reconnection lead to inconsistent contact resistance and clamping force. |
In ex-situ testing, the significant fluctuations in Ohmic resistance (Rs) are primarily caused by connection noise, which masks the real changes in the intrinsic resistance of the electrolyte, separator, and other components, reducing the reliability of the data. |
| State Consistency |
Excellent. In-situ EIS is automatically performed after a strictly controlled resting period following charge and discharge, accurately locking the target SOC point. |
Poor. During the disassembly, transfer, and waiting periods for testing, the battery experiences uncontrollable self-discharge and state relaxation, causing its SOC to deviate from the target point. |
Ex-situ testing cannot guarantee that each measurement is taken under identical conditions, which can lead to distortion of the charge transfer resistance (Rct) and Warburg diffusion impedance (Zw) data, rendering it unsuitable for precise kinetic analysis. |
| Efficiency |
High. It enables measurements at specific stages of each charge-discharge cycle, providing impedance evolution data with ultra-high temporal resolution. |
Low. The process is cumbersome and time-consuming, typically only allowing measurements at cycle interval points (e.g., every 50 cycles), which may miss critical aging inflection points. |
In-situ EIS can capture non-linear transitions during battery aging (such as SEI fracture/reformation and lithium plating), providing robust data support for failure mechanism research while significantly enhancing scientific research efficiency. |
This comparative experimental study finds that the in-situ EIS testing method provides more accurate and reliable impedance data. By enabling real-time monitoring of the battery under actual operating conditions, it effectively avoids the additional errors introduced by battery transfer, environmental changes, and manual operations inherent in ex-situ testing, thus more authentically reflecting the internal electrochemical kinetic processes. Therefore, impedance data obtained using in-situ EIS can provide a solid and effective basis for in-depth analysis such as battery state of health assessment, failure mechanism analysis, model parameter fitting, and lifetime prediction, enhancing the scientific validity and reliability of research outcomes.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.
Please fill out the form below and we will contact you asap!


