-
iestinstrument
Study on the Mechanism of Battery Binders on Electrode Tortuosity and Performance Regulation
1. Background
As one of the most important electrochemical energy storage devices today, the performance enhancement of lithium-ion batteries has always attracted significant attention. Traditional research often focuses on the improvement of active materials, while overlooking the importance of the “unsung hero” – the binder. Although comprising only 2%-5% of the electrode mass, battery binders have a decisive influence on the electrode’s microstructure and electrochemical performance. This article systematically analyzes the multiple action mechanisms of battery binders within the electrode, explores their regulatory mechanism on electrode tortuosity and its impact on battery performance, providing new insights for the development of next-generation high-performance batteries.
2. Regulatory Mechanism of Binders on Electrode Microstructure
2.1 Battery Binder-Adhesion Mechanism
There are two main types of bonding mechanisms between binders and active materials:
-
Indirect Bonding: In this mechanism, binders interact with active materials primarily through non-covalent bonds. The binder forms a weak van der Waals force with the active material, resulting in a limited bonding strength.
-
Direct Bonding: In this mechanism, binders form covalent bonds with the active material, creating a cohesive structure. This method requires less binder and provides stronger bonding, making it more suitable for high-energy-density batteries.
2.2 Binder-Particle Interface Characteristics
The interaction between the binder and active material’s interface plays a crucial role in ion transport:
-
Wettability: Hydrophilic binders (e.g., CMC) improve electrolyte wetting on the electrode, reducing interfacial impedance.
-
Chemical Stability: Some battery binders (e.g., PAA) can form a stable passivation layer on the active material’s surface, inhibiting side reactions but may also increase the resistance to ion transport at the interface.
-
Mechanical Compatibility: During charging and discharging, the binder needs to adapt to the volume changes of active materials. Rigid binders may cause interfacial failure, whereas elastic binders maintain good interface contact.
3. Mechanism of Binder Regulation on Electrode Tortuosity
3.1 Relationship Between Binder Content and Curvature
-
Low Content Region (<2 wt.%): The binder is insufficient to form a continuous network, leading to poor particle contact and the formation of large but poorly connected pores, which increases the curvature.
-
Optimal Content Region (2-4 wt.%): The binder forms an ideal three-dimensional network that stabilizes the particles while maintaining sufficient interconnected pores, resulting in minimal curvature.
-
High Content Region (>5 wt.%): Excessive binder severely blocks the pores, significantly increasing the curvature.
3.2 Effect of Battery Binder Type on Tortuosity
-
PVDF-based Binders: Due to hydrophobicity and strong film-forming ability, they tend to form high-tortuosity structures. Their advantage lies in high mechanical strength, suitable for high-energy-density electrodes.
-
Water-based Binders (CMC/SBR): Good hydrophilicity and excellent electrolyte wettability typically result in lower tortuosity. However, mechanical strength is relatively weaker.
-
Composite Binders: Combine the advantages of different binder types, maintaining high strength while potentially reducing tortuosity.
4. Binder-Curvature-Battery Performance Correlation Mechanism
4.1 Rate Performance Regulation
-
Low-Tortuosity Electrodes: With unobstructed ion transport paths and low polarization, these electrodes are suitable for high-rate charge-discharge.
-
High-Tortuosity Electrodes: Ion transport is limited, and high currents lead to significant polarization, reducing the rate performance.
4.2 Cycle Stability Impact
-
Structural Stability: Adequate binder content helps maintain the electrode structure, preventing particle shedding during cycling. However, excessive binder leading to high curvature can exacerbate local polarization, accelerating capacity fade.
-
Interface Stability: Electrodes with low curvature exhibit more uniform ion distribution, reducing the risk of overcharge/overdischarge, thereby extending cycle life.
5. Electrode Tortuosity Testing
As mentioned earlier, accurately measuring electrode sheet curvature is an essential parameter for evaluating binder performance and optimizing electrode design. How can we efficiently and accurately test electrode sheet curvature? IEST self-developed multi-channel ion conductivity device, through assembling symmetric cells and testing electrochemical impedance spectroscopy (EIS), can quickly obtain curvature data that truly reflects the microstructure of the electrode sheets. This method is straightforward and avoids the errors inherent in traditional indirect characterization, providing reliable technical support for battery binder screening and electrode process optimization.
5.1 Test Equipment
Electrode Tortuosity Testing: The multi-channel ionic conductivity test system (EIC2400M, IEST) is shown in Figure 1. This device contains 4 test channels, provides a high-purity argon atmosphere, and enables electrochemical impedance spectroscopy (EIS) testing on multiple symmetric cells simultaneously. Pressure range: 10-50 Kg, Frequency range: 100 KHz – 0.01 Hz.
Figure 1. IEST Multi-channel Ionic Conductivity Test System
5.2 Test Samples
Graphite anode sheets prepared with different binders.
5.3 Results Analysis
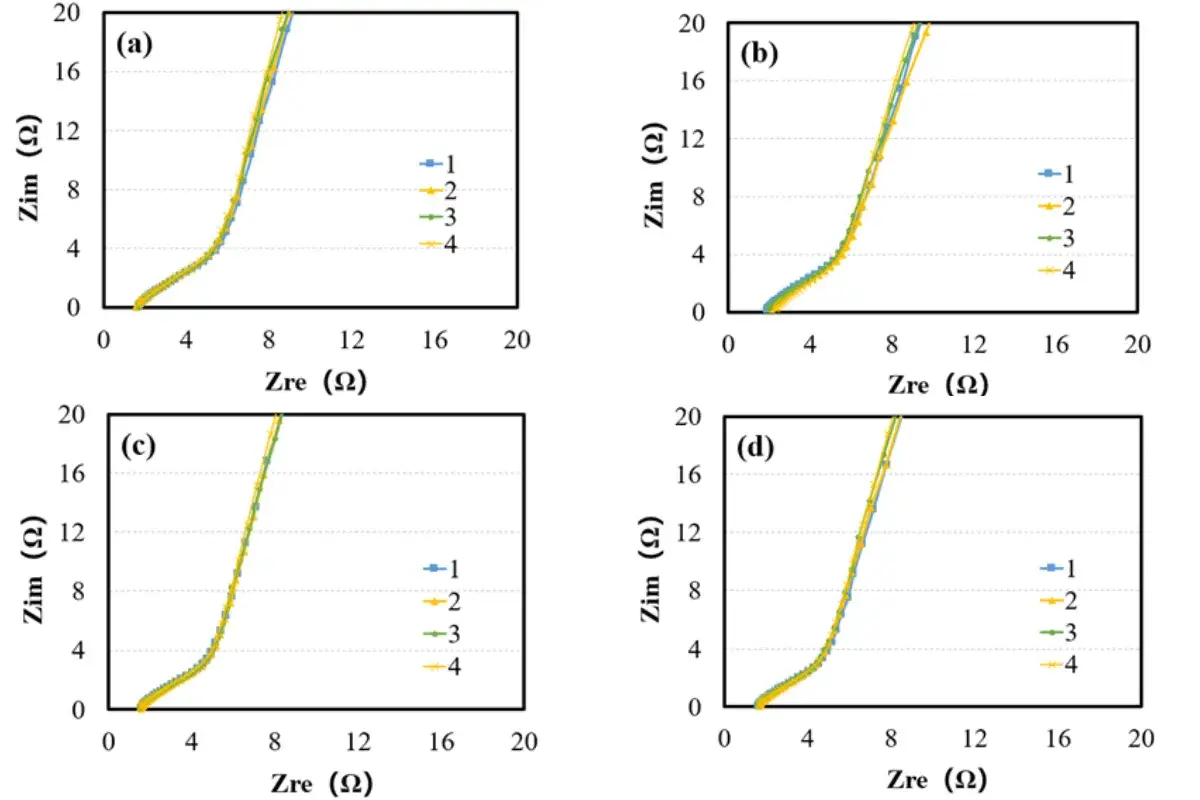
Figure 2. Nyquist plots of symmetric cells with electrodes using different binders: A (a); B (b); C (c); D (d)
Table 1. Ionic Resistance and MacMullin Number of Electrodes with Different Battery Binders
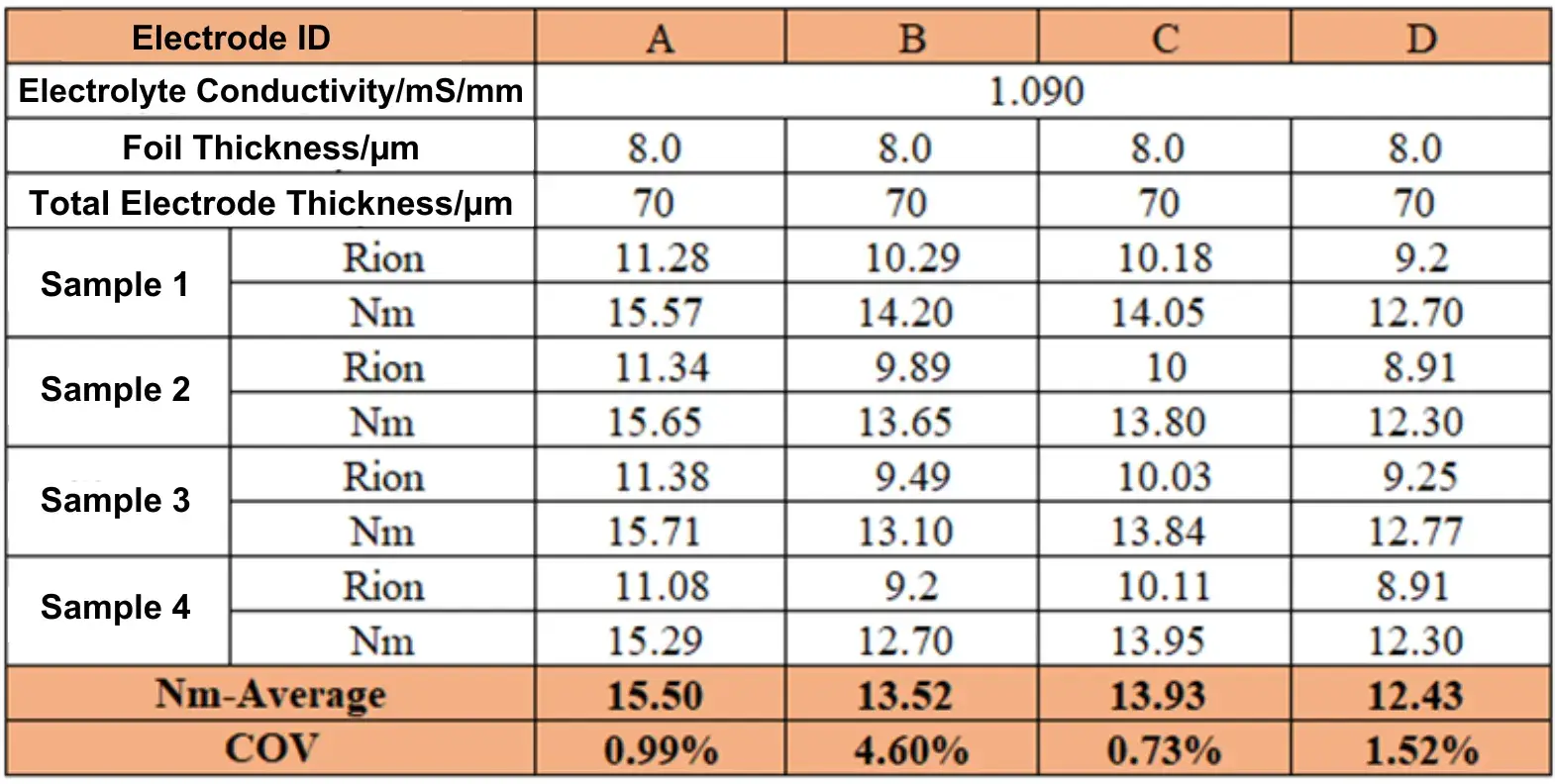
Symmetric cells assembled with anodes prepared using different binders were subjected to EIS testing; the results are shown in Figure 2. The EIS spectra were fitted and calculated to obtain the ionic resistance and MacMullin number (a direct indicator of tortuosity) for each electrode, as listed in Table 1. The data shows the order of tortuosity magnitude: Electrode A > C > B > D.
Experimental data indicate that the tortuosity of electrodes varies with the binder used. The type of binder has a critical influence on the electrode microstructure (e.g., pore connectivity, ion transport paths). Tortuosity, as an important parameter for evaluating electrode performance, its variation can indirectly reflect the influence of the binder on electrode electrolyte wettability and lithium-ion diffusion efficiency, thereby correlating with the cell’s rate capability, internal resistance, and cycle stability. Therefore, measuring tortuosity enables rapid screening of binders and optimization of electrode design, providing a basis for enhancing overall battery performance.
6. Summary
This article systematically summarizes the regulatory role of battery binders on electrode microstructure and tortuosity, revealing the influence of binder type, content, and distribution uniformity on the pore network and ion transport paths. Through the electrochemical impedance spectroscopy (EIS) testing method, efficient characterization of electrode tortuosity was achieved, providing a reliable basis for binder performance evaluation and electrode design optimization. Optimizing the binder system can improve the battery’s rate capability and cycle life, holding significant guiding importance for the development of high-performance lithium-ion batteries.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



