-
iestinstrument
The Impact of PAA Binder on the Electrical Conductivity and Compression Performance of Silicon-Based Anodes
1. Abstract
This study utilizes the IEST Electrode Sheet Resistance Tester (BER2500) to conduct a comparative evaluation. We systematically assess the electrical conductivity and compression performance of Si/C anode sheets prepared with CMC/SBR versus PAA binders, quantifying the impact of binder choice on these critical electrode properties.
2. The Silicon Anode Promise and the Binder Challenge
In recent years, with the continuous development of the new energy industry, the specific capacity of graphite anodes can no longer meet the future demands for battery energy density. In contrast to graphite, silicon possesses an ultra-high theoretical specific capacity of 4200 mAh·g⁻¹ in its fully lithiated state. This means that under the same mass conditions, batteries with silicon anodes can store significantly more capacity than those with graphite anodes. However, during cycling, the insertion of lithium ions causes a massive volume expansion effect (~300%) in silicon anodes. As lithium ions are repeatedly inserted and extracted, the volume of silicon continuously changes. This intense volumetric fluctuation leads to crack formation on the electrode surface. Furthermore, these propagating cracks cause electrode fragmentation and pulverization of silicon particles. Ultimately, this results in the detachment of active material from the current collector, disruption of the conductive network, continuous capacity loss, and complete battery failure.
To mitigate the adverse effects of this volume change, three primary strategies are currently mainstream [1~4]: (1) Structural design of silicon, such as preparing silicon nanotubes, nanowires, or nanoshells; (2) Fabrication of silicon-based composite materials (e.g., silicon-carbon anodes, silicon oxide-carbon anodes) to alleviate volume expansion through synergistic effects; (3) Synthesis of high-performance lithium-ion battery binders to suppress silicon’s volume expansion. Designing silicon structures often involves high costs and complex processes, largely remaining at the laboratory stage. Therefore, preparing silicon-based composites and employing high-performance binders are currently the more widely adopted methods.
3. PAA vs. CMC/SBR: A Mechanistic Comparison for Binder Selection
For silicon-based anodes, PAA binder has become a subject of intense research[5~6]. As a water-soluble, linear polymer, PAA offers distinct advantages over conventional carboxymethyl cellulose / styrene-butadiene rubber (CMC/SBR) systems. The key difference lies in the bonding mechanism. PAA facilitates “segment-to-surface” bonding, creating a more uniform, cohesive network that anchors particles more effectively, leading to greater electrode integrity. In contrast, SBR/CMC typically provides “point-to-point” bonding. This structural superiority of PAA translates to minimal swelling in carbonate-based electrolytes and excellent structural stability during cycling. Furthermore, the abundant carboxyl (-COOH) groups in PAA can form strong hydrogen bonds with functional groups on the silicon and carbon surfaces. This promotes a more uniform coating, enhances adhesion to the current collector, and contributes to forming a more stable Solid Electrolyte Interphase (SEI), collectively improving cycle performance.
4. Experimental Methodology: Precision Measurement of Electrode Properties
4.1 Test Equipment
The core instrument is the BER2500 Electrode Sheet Resistance Tester (see Figure 1). It accommodates electrode samples with a 14 mm diameter and applies pressure within a range of 5 to 60 MPa. The system simultaneously collects key parameters including sheet resistance, resistivity, electrical conductivity, and compaction density in real-time.
Figure 1. (a) BER2500 appearance diagram; (b) BER2500 structural diagram.
4.2 Experimental Procedure
Two sets of Si/C anode sheets were prepared with identical formulations and active materials, differing only in the binder system: one with CMC/SBR (labeled SC-CMC) and one with PAA (labeled SC-PAA).
-
Electrode Resistance Test: A single-point test mode was employed at a constant pressure of 5 MPa with a 15-second hold. Eight data points were sampled per sheet to obtain average values for thickness, resistance, resistivity, and conductivity.
-
Compression Performance Test: A steady-state test mode was used, ramping pressure from a lower limit of 5 MPa to an upper limit of 60 MPa in 5 MPa steps, with a 15-second hold at each step. This profile measures the electrode’s thickness change andcompression performance under varying pressure.
5. Results & Discussion: Electrical Conductivity Advantage of PAA Binder
The comparative data for sheet resistance, resistivity, and thickness is presented in Figure 2. The results clearly indicate that the Si/C anode sheet prepared with the CMC/SBR binder exhibits slightly higher resistance and resistivity than its PAA-based counterpart.
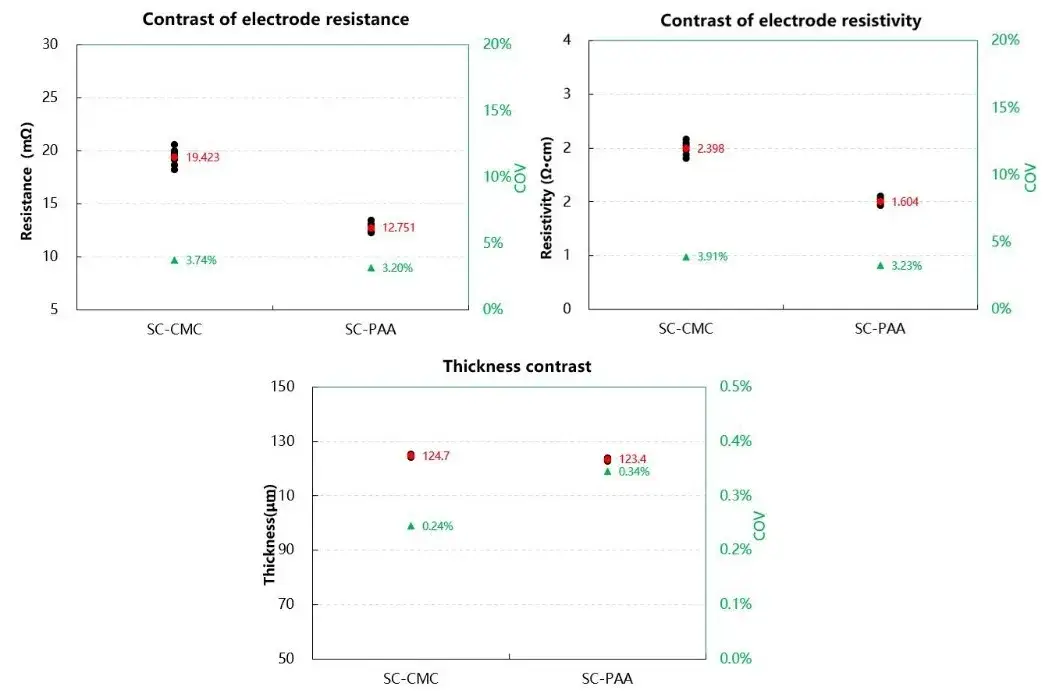
Figure 2. Comparative test results for resistance, resistivity, and thickness of the two electrode sheets.
This enhancement in electrical conductivity with PAA binder can be attributed to its bonding mechanism. As illustrated in Figure 3, PAA’s linear structure enables more extensive “segment-to-surface” contact with active material particles compared to the “point-to-surface” contact of SBR latex. This superior adhesion, driven by strong hydrogen bonding with surface hydrated layers, fosters a more uniform coating over silicon particles. Additionally, the high concentration of polar groups (e.g., sodium carboxylate) in PAA improves bonding to the current collector. This dual effect strengthens the electrical contact network both between active material/conductive agent particles and between the composite and the current collector, thereby reducing overall electronic resistance. Research also suggests that PAA may participate in SEI formation, where its -COOH groups can interact with solvated Li⁺ ions, potentially facilitating desolvation and lithium-ion insertion kinetics.
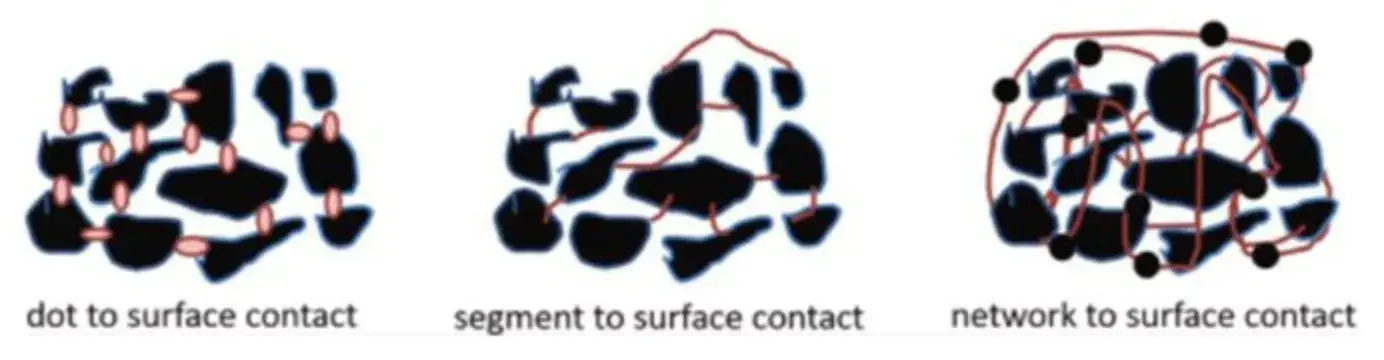
Figure 3. Schematic of binder-active powder interactions: point-to-surface, segment-to-surface, and network-to-surface contact.
6. Results & Discussion: Superior Compression Performance of PAA
The compression performance test results are shown in Figure 4 and summarized in Table 1. Key metrics like maximum deformation, reversible deformation, and irreversible deformation were all greater for the SC-CMC electrode compared to the SC-PAA electrode.
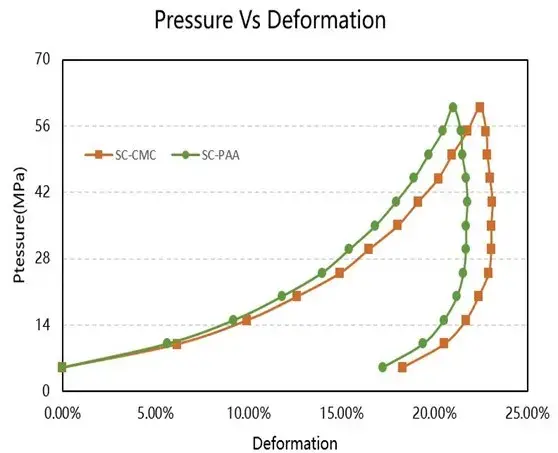
Figure 4. Comparative compression performance test results for the two electrode sheets.
Table 1. Summary of compression performance data for the two electrode sheets.
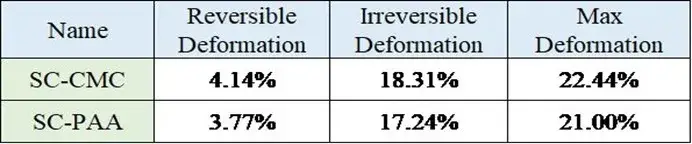
This indicates that the CMC/SBR-based electrode is more compressible. A significant downside of this higher compressibility is greater elastic rebound (spring-back) after calendering, which poses challenges for cell assembly and precise control of electrode porosity. The difference stems from the inherent mechanical properties of the binders. CMC possesses a rigid cellulose backbone, imparting stiffness and brittleness. While SBR is added as an elastomeric modifier to reduce brittleness and increase maximum strain, the composite still shows different viscoelastic behavior compared to PAA. The polyethylene-like backbone of PAA offers greater flexibility, contributing to its lower permanent deformation and reduced rebound under pressure.
7. Conclusion: PAA Binder as a Key Enabler for Robust Silicon Anodes
This case study demonstrates the utility of precise electrode-level testing in binder selection for silicon-based anodes. Using the BER2500 system, we quantitatively compared CMC/SBR and PAA binders. The results confirm that the PAA binder not only improves electrical conductivity by enhancing inter-particle and electrode-current collector contact but also offers superior compression performance characterized by reduced elastic rebound after calendering.
These benefits are primarily due to PAA’s chemical structure. Its higher carboxyl group content facilitates stronger hydrogen bonding with active materials, promoting a more homogeneous coating. This leads to a more stable electrode architecture that can better accommodate volume changes during cycling, fosters a more robust and thin SEI layer, reduces interfacial impedance (particularly charge transfer resistance), and improves lithium-ion diffusion rates. Consequently, selecting an advanced binder like PAA is a crucial step in developing high-performance, durable silicon-based lithium-ion batteries.
8. References
[1] Kang K, Song K, Heo H, et al. Kinetics-driven high power Li-ion battery with a-Si/NiSix core-shell nanowire anodes[J]. Chemical Science, 2011, 2(6): 1090-1093.
[2] FU Yan-peng,CHEN Hui-xin,YANG Yong . Silicon Nanowires as Anode Materials for Lithium Ion Batteries[J]. Journal of Electrochemistry,2009,15(1): 54-61.
[3] Zhang X, Wang D, Qiu X, et al. Stable high-capacity and high-rate silicon-based lithium battery anodes upon two-dimensional covalent encapsulation[J]. Nature Communications, 2020, 11(1): 3826.
[4] Gendensuren B, He C, Oh E-S. Preparation of pectin-based dual-crosslinked network as a binder for high performance Si/C anode for LIBs[J]. Korean Journal of Chemical Engineering, 2020, 37(2): 366-373.
[5] Magasinski A., Zdyrko B., Kovalenko I., et al. Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid[J]. Acs Applied Materials & Interfaces, 2010, 2(11): 3004-3010.
[6] Komaba S., Shimomura K., Yabuuchi N., et al. Study on Polymer Binders for High-Capacity SiO Negative Electrode of Li-Ion Batteries[J]. Journal of Physical Chemistry C, 2011, 115(27): 13487-13495.
[7] Ma Y , Ma J , Cui G . Small things make big deal: Powerful binders of lithium batteries and post-lithium batteries[J].Energy Storage Materials, 2019, 20: 146-175
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



