-
iestinstrument
Evaluation of Electrical Conductivity and Compression Properties of NCM Materials in Different Systems
The cathode material is one of the most important components of lithium ion batteries. The common cathode materials of lithium ion batteries are layered lithium cobalt oxide, olivine structure lithium iron phosphate, lithium manganese oxide of spinite structure and layered nickel-cobalt-manganese ternary material (hereinafter referred to as NCM).
Among them, NCM materials have the characteristics of lithium cobalt oxide, lithium manganese oxide and lithium nickel acid, which weaken their shortcomings to a certain extent, with the advantages of low cost, small environmental pollution, low toxicity, high energy density and high voltage platform. Therefore, NCM materials have quickly become an important direction for the development of lithium ion battery materials.There is a synergistic interaction between nickel, cobalt and manganese in NCM materials, and its structure is generally LiNixCoyMnzO2(x + y + z=1), which is a hexagonal and layered structure.
The presence of nickel can improve the specific capacity of material, reduce material cost, but too high nickel content will cause material structure instability and mixed nickel lithium; cobalt improves the electronic conductivity and multiplier performance of the material, but cobalt toxicity, and cobalt resources; manganese plays the role of stabilizing material structure and reducing material cost, but too high is easy to produce spinel phase and destroy the layered structure of the material. Three NCM polycrystalline materials (NCM111, NCM622, and NCM811) with different nickel content were selected to evaluate the differences between materials by measuring their morphology, conductivity, compaction density, and rebound properties.

Figure 1. LiCoxMnyNi1-x-yO2crystal structure4
1. Test Method
1.1 The SEM Morphology Test of the Three Materials
The electrical conductivity and compaction density of the three NCM materials are tested by using PRCD3100(IEST). The test equipment is shown in Figure 2.
Test Parameters: Apply a pressure range of 10-200MPa, with 20MPa interval, and pressure holding for 10s.
Figure 2. (a) PRCD3100 appearance diagram; (b) PRCD3100 structure diagram
2. Test Results
For SEM morphology test of the three NCM materials, the results are shown in Figure 3, (a), (b), (c), NCM111, NCM622, NCM811 material morphology, the three kinds of material found mixed particles, and NCM811 surface density is higher, the structure is closer to the spherical, NCM111, and NCM622 morphology, low surface density and layered structure is more obvious.The conductivity and compressive properties of ternary materials, on the one hand, the conductivity and elasticity of the particles themselves, and on the other hand, the contact resistance and porosity caused by differences in particle size distribution.

Figure 3. The SEM topography of the three NCM materials
The conductivity and compaction density test curves of the three NCM materials are shown in Figure 4. From the conductivity result curve (a), the conductivity size is NCM811> NCM622> NCM111, with the conductivity of the three NCM materials and the compaction density curve (b), the compaction density of NCM622 above about 80MPa is greater than the other two NCM materials, but the overall difference is not large.
The compaction density of the positive electrode materials is related to the particle shape, particle size and its distribution. If the deformation of the particles themselves is not considered first, the compaction process of the powder particles is the process in which the powder forms the densest accumulation from the loose state under the action of pressure. According to the most compact packing principle, when the spherical particles with radius R accumulate in the most compact way, all the particles contact with each other, the theoretical porosity formed between the particles is 25.94%, and the pores between the primary particles with radius R is 0.414R. After all the pores are filled with secondary particles, the porosity is 20.70%. The maximum particle radius that can be refilled in the pore is 0.225R, cubic, 0.177R, and 0.116R, corresponding to a theoretical porosity of 19%, 15.8%, and 14.9%.
When all the particles between the closest accumulation, further pressure, mutual deformation between the particles itself, the first accumulation of elastic strain, pressure unloading elastic strain rebound back, when the force is greater than the yield strength of the material itself particles plastic deformation, strain can not recover. Therefore, the compaction density of the particles is not only related to the morphology and particle size of the powder, but also related to the mechanical properties of the powder. Therefore, the deformation behavior under pressurization and unloading was further tested.
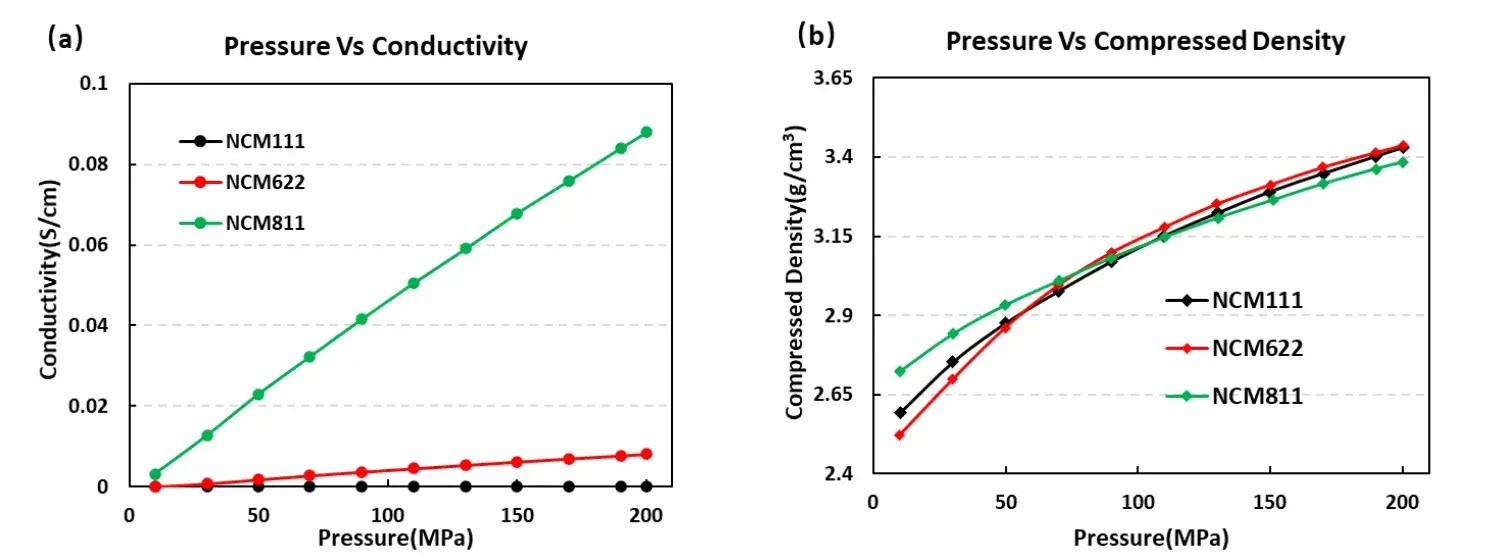
Figure 4. Electrical conductivity and compaction density curves of the three NCM materials
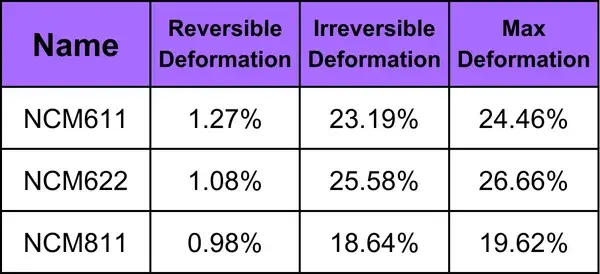
Test the pressure and pressure relief of the three materials, and test the loading pressure according to the pressure change curve shown in Figure 5 (a), the corresponding material thickness change and the thickness rebound curve are shown in Figure 5 (a) and (b). When the three NCM powders under the same sampling amount, the thickness rebound of NCM622 is greater than NCM111 and NCM811 materials. At about 110MPa, the thickness rebound amount gradually stabilizes, indicating that the pores between the particles are basically excluded, and the thickness rebound is mainly due to the elasticity of the particles themselves. In addition, using the continuous pressure relief in Figure 5 (c) and maximum pressure relief in Fig. 5 (d), by analyzing the maximum form variable, reversible form variable and irreversible form variable, as shown in Table 1, NCM111 reversible deformation is slightly greater than NCM622 and NCM811, but the overall difference is not large. From the slope of the stress strain curve, the compression modulus of NCM622 is easier because it is less than NCM111 and NCM811.The above test results can indicate that the NCM622 selected in this experiment can achieve a higher compaction density compared to the other two NCM materials.
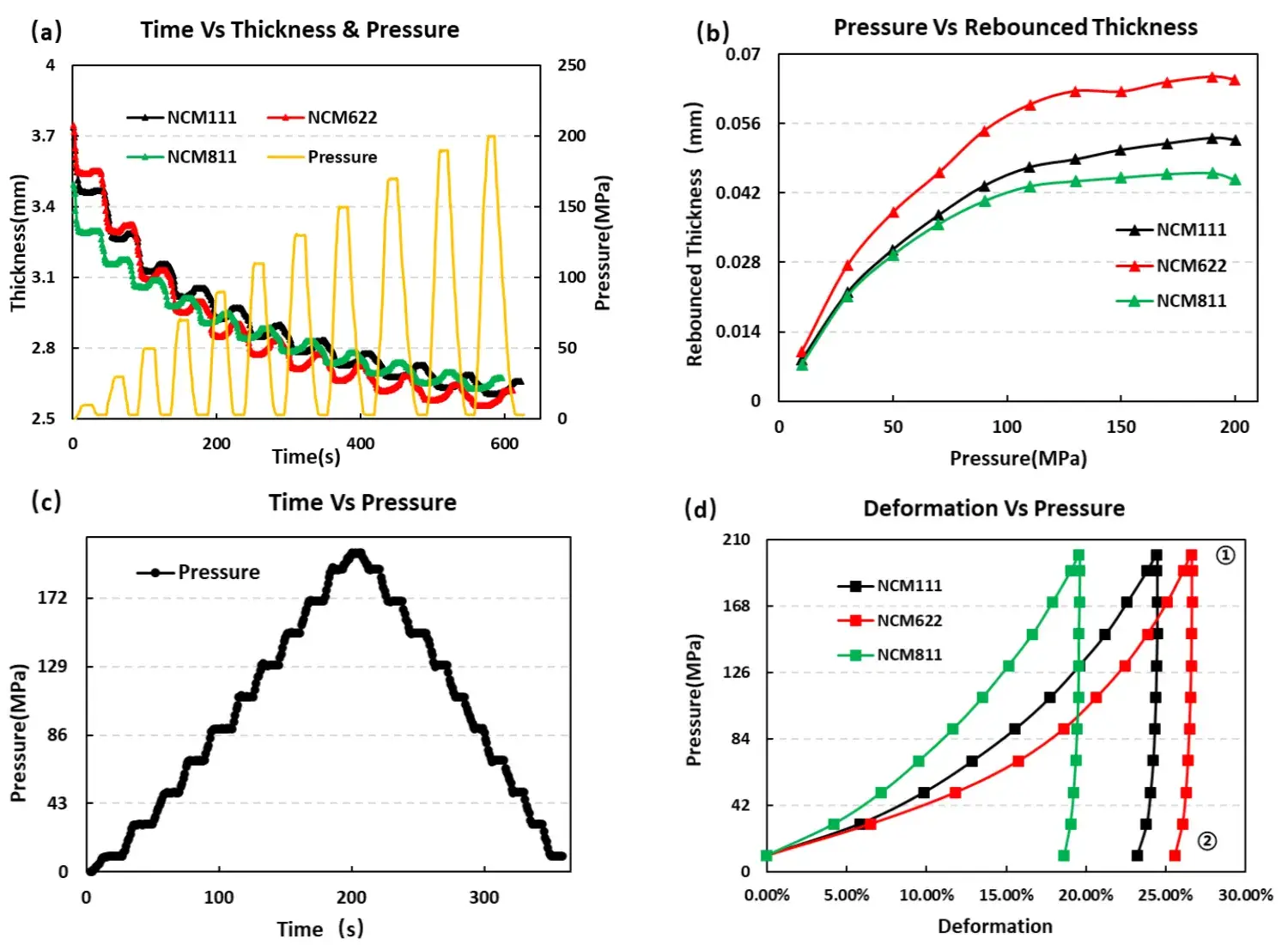
Figure 5. Stress and strain curve during the pressurized pressure relief of the three NCM materials
Table 1. Summary of Shape Variable Data for the Three NCM Materials
3. Summary
In this paper, PRCD3100 tests the conductivity, compaction density and compression properties of NCM111, NCM622 and NCM811, and finds that with the increase of nickel content, the compression modulus of NCM622 is greater than NCM111 and NCM811, which is related to the microstructure. Combined with the SEM morphology analysis results of the three materials, it is obvious that the polycrystalline material composed of layered structure is easier to be compressed, which is consistent with the actual compression performance experiment results. In the process of lithium battery development, in addition to by increasing the nickel content to improve battery energy density, also can be on the premise of ensuring no broken particles increase pole sheet compaction density to improve volume energy density, and the material conductivity and compaction density will affect cell performance, therefore need to develop personnel to comprehensively evaluate the influence of the nickel content.
4. References
[1] Zhang R, Meng Z, Ma X, et al. Understanding fundamental effects of Cu impurity in different forms for recovered LiNi0.6Co0.2Mn0.2O2 cathode materials[J].Nano Energy, 2020:105214.
[2] Guangshun Xiao. Recent Development on Ni-Co-Mn Ternary Cathode Material for Lithium-Ion Batteries[J].Material Sciences, 2020, 10(4):201-215.
[3] Tang Zhongfeng. Synthesis, characterization and modification of high nickel ternary cathode materials for lithium-ion batteries [D]: [Doctoral dissertation]. Hefei: University of Science and Technology of China, 2018.
[4] Meng Y S, Arroyo-De Dompablo M E. Recent advances in first principles computational research of cathode materials for lithium-ion batteries[J].Accounts of Chemical Research, 2013, 46(5):1171-80.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.