-
iestinstrument
In situ Gas Production Analysis Of Cell Anti-overcharge Electrolyte Additive
1. Preface
When lithium-ion battery is overcharged, too many lithium ions take out out of the cathode material, and the voltage and temperature of the battery rise rapidly, releasing a large amount of oxygen and heat. When a certain potential is reached, the electrolyte will undergo oxidation decomposition, violent chemical reaction, produce a large amount of heat, and then may be dangerous. The anti-overcharge electrolyte additives, can early warning in the battery overcharged behavior, prevent the failure of active materials, including biphenyl (BP) as a type of electric polymer anti-overcharge electrolyte additive, can be charged in the battery to above 4.5V, forming a layer of polymer membrane, produce a large amount of gas, increase the battery resistance, the function of early warning battery overcharge.
In this paper, the in-situ gas volume monitor (GVM) was used to test the NCM523 / graphite cell (theoretical capacity of 1000mAh) of the electrolyte system with different contents of BP, and the cell gas production behavior was relatively analyzed, and the influence of BP addition on the gas production composition was analyzed according to gas chromatography (GC).
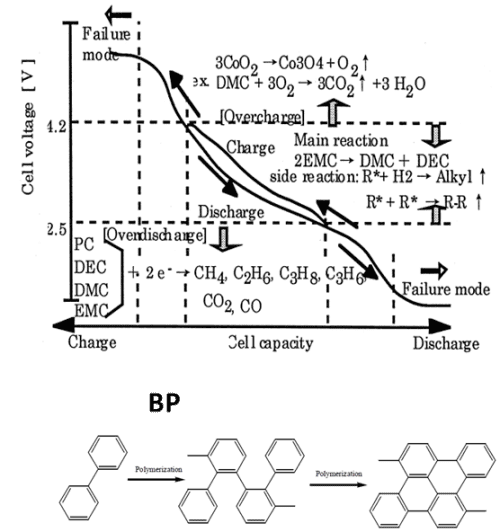
Figure 1. Schematic diagram of the gas production behavior of different electrolyte decomposition and the electropoly merization reaction of BP.
2. Experimental Equipment and Test Methods
2.1 Experimental Equipment
Model GVM2200(IEST) , test temperature range of 20℃~85℃, support dual-channel (2 cells) synchronous test, the appearance of the equipment as shown in Figure 2.
Figure 2. Appearance of GVM2200 Equipment
2.2 Test Parameters: 25℃, 1C CC to 5V.
2.3 Test Method
Initially weighing the cell m0, put the cell to be tested into the corresponding channel of the equipment, open the MISG software, set the corresponding cell number and sampling frequency parameters of each channel, and the software will automatically read the volume change, test temperature, current, voltage, capacity and other data. The gas composition test uses a GC-2014C gas chromatography instrument, removing 1mL of gas from the overcharged cell in the glove box, and testing different types of gas concentrations using TCD and FID detectors, respectively. The measurable gas types are shown in Figure 3.

Figure 3. Gas composition that can be tested by FID and TCD detectors
3. Analysis of In-situ Overcharge Gas
3.1 Analysis of Charge and Discharge Curve and Volume Change Curve
The volume and voltage changes of the cell are shown in Figure 4 (a) and (b), and the B P content in the electrolyte is 0%, 1%, 2%, 3% and 5%, respectively. It can be seen from the curve that, with the increase of the electrolyte additive content, the volume change of the cell is getting bigger and bigger, indicating that the cell is swollen due to the gas production reaction of the additive. When the content of electrolyte additives reaches 5%, it can be seen from the voltage curve that the cell voltage is difficult to reach the upper limit of 5V. At this time, the additive of the NCM positive electrode first occurs an electrochemical polymerization reaction, forming a layer of polymer deposition on the particle surface, and then the deposition layer gradually grows with the continuous reaction of the electrolyte additive. The reaction process stably maintains the battery voltage at the electropolymerization potential of the additive, and the voltage platform is shown in Figure 4a.
As overcharging continues, large amounts of polymeric deposits accumulate and penetrate the diaphragm, eventually forming a direct connection between the two electrodes. Because of the polymerization products of biphenyl (such as poly (biphenyl)) are electronically conductive, the conductive bridge formed by the polymerization sediment in the two electrodes will cause an internal short circuit in the battery, which continues to consume the charging current, thus preventing the battery voltage out of control and the voltage cannot reach 5V. The internal short circuit caused by electropolymer growth is a slow process, and occurs only on some part of the particle surface, which produces a moderate self-discharge to automatically discharge the battery to a safe state, and the process does not cause intense thermal runaway of the battery. As shown in Figure 4a, the battery voltage gradually decreases with the self-discharge inside the battery.
To the cell total gas and gas starting potential analysis, when no BP, the cell gas starting point almost close to 5V, the overcharge voltage, is likely to cause the destruction of the cathode material structure, and after the BP, the cell gas production voltage between 4.5~4.6V, this is the voltage range of BP, and with the increase of BP concentration, the total cell production also increased from about 2mL to about 10mL, can be combined with the explosion-proof safety valve to achieve the effect of early warning.
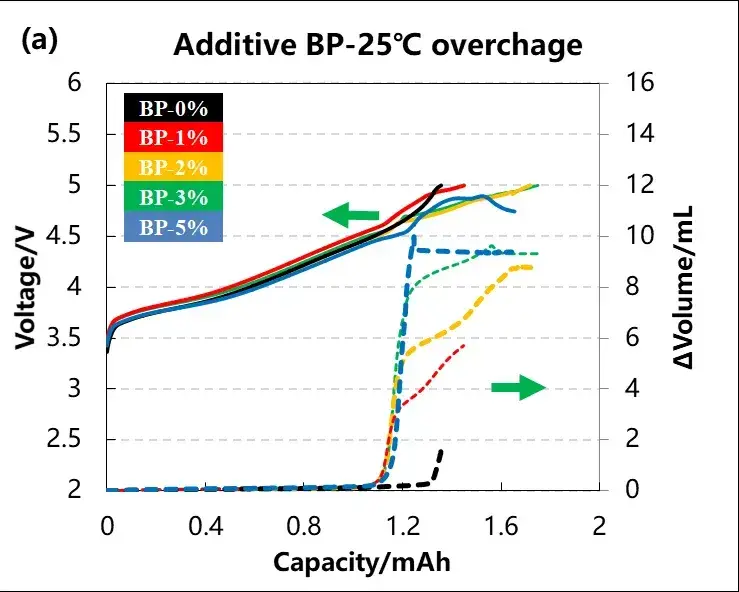
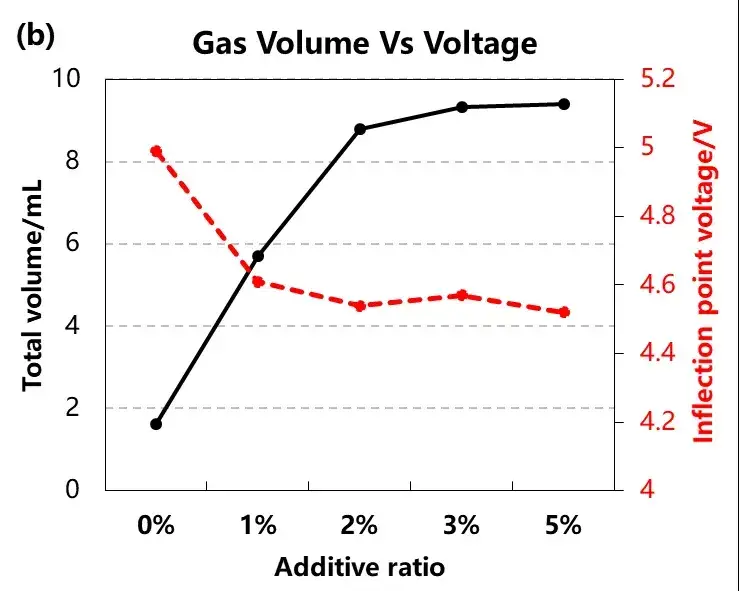
Figure 4. Charge-discharge and volume changes of different amounts of BP
3.2 Analysis of the Gas Production Composition of the Battery Cell
After overcharged gas cells, 1mL of gas, gas qualitative analysis, as shown in figure 5, we found that with the increase of BP concentration of gas, the concentration of methane CH4 gradually decreased, and the concentration of hydrogen H2 gradually increased, other components such as oxygen O2, ethylene C2H4, carbon monoxide CO have no obvious change trend.
Further combined with the reaction mechanism analysis, BP biphenyl (BP) as a type of electric polymerization anti-overcharge electrolyte additive, in the battery charging above 4.5V, monomer molecules in the electrolyte oxidation into radical ions, these radical ions even synthesis polymer in the electrolyte, and deposited in the positive and near the positive diaphragm surface, the electrode isolation, increase battery resistance, at the same time will be accompanied by a large amount of hydrogen release, lead to cell gas, the function of early warning battery overcharge.
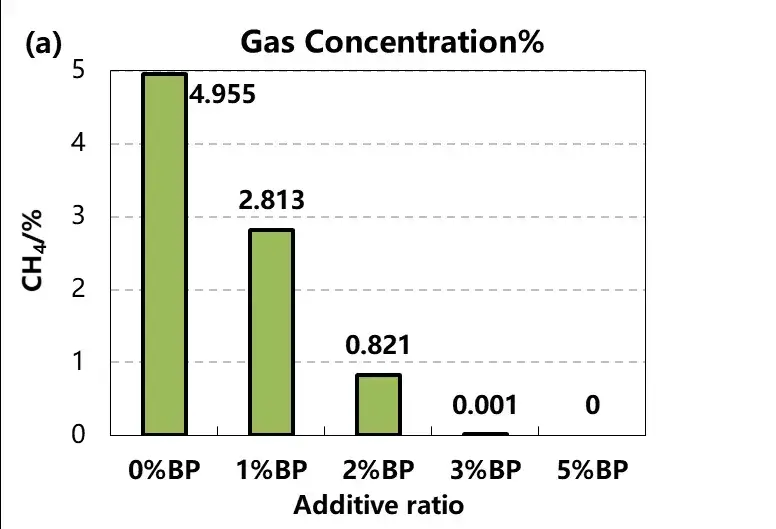
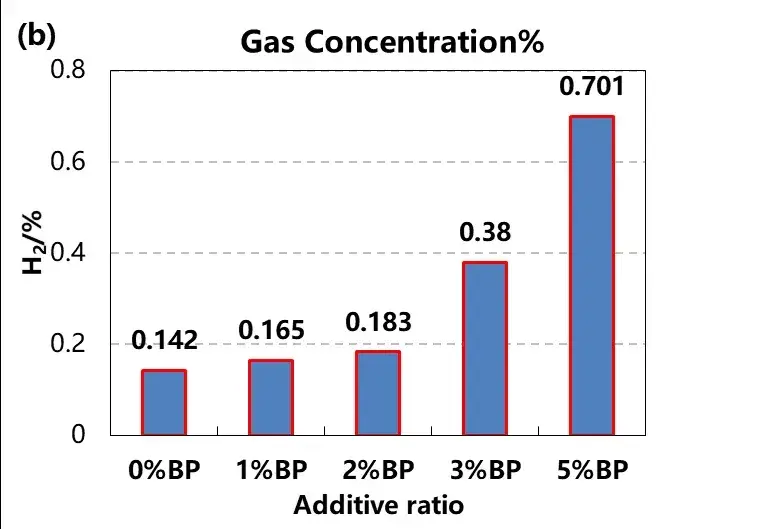
Figure 5. Change of methane and hydrogen concentration in the cell gas with different electrolyte additive content
4. Summary
This paper adopts a controllable temperature dual-channel in-situ battery gassing volume analyzer (GVM), and combined with the gas chromatography, the qualitative and quantitative analysis of the anti-overcharge electrolyte additive BP gas behavior, not only provides the different content of BP electrolyte gas potential, and proved that the electrolyte additive is in the process of overcharge generate a lot of hydrogen makes the cell gas, thus can realize the function of early warning cell overcharge.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



