-
iestinstrument
Analysis of Swelling Behavior in NCM Cathode Materials
1. Background
As we all know, lithium-ion batteries will experience structural swelling and contraction during de-lithium extraction/intercalation. For anode electrode materials, whether it is intercalated lithium intercalation of graphite or alloyed lithium intercalation of silicon-based anode electrodes, the common feature is that when lithium is intercalated, a relatively obvious volume expansion occurs,however the volume shrinks obviously when detached, which is consistent with conventional cognition. During the swelling test of the pouch cell, we will find that the pouch cell of some systems (especially the high-nickel ternary system) will change from volume expansion to volume shrinkage at the end of charging, in the initial stage of discharge, it will expand first and then shrink, that is, it will show an “M” type swelling behavior under high voltage. This “M” type of swelling behavior is most likely caused by the cathode electrode, so this also prompts us to focus more attention on the research on the swelling behavior of the cathode materials.
2. Comparison of Swelling Results
We selected two ternary cathode materials with different Ni contents, NCM111 and NCM622, and assembled them into button-type full batteries (the anodes are all conventional graphite materials) to test the swelling thickness during the cycle charge and discharge process, the test equipment is IEST silicon-based anode electrode swelling in-situ rapid screening system (RSS1400, as shown in Figure 1(a)), and the test results of the swelling thickness are shown in Figure 1(b). It can be seen from the figure that for the button-type full battery whose cathode electrode is NCM111, it expands monotonously with charging, and shrinks monotonically when discharged; However, for the NCM622 cathode electrode with a higher Ni content, its swelling and contraction are not monotonous. It will expand first during charging, but it will show a contraction behavior in the high voltage region at the end of charging, this non-monotonic swelling behavior is reversible during discharge, that is, the volume swelling occurs at the beginning of the discharge, and then turns into volume contraction. Under three cycles, the NCM622 system exhibits such “M” type swelling behavior, indicating that this swelling behavior is the intrinsic behavior of high Ni cathode materials. To study this “M” type swelling behavior related to the Ni content in detail, we consulted the relevant literature to analyze the microscopic mechanism of this swelling behavior from in-situ XRD and lattice parameters. See the “Results Analysis” section of this paper for more details
![]()
Figure 1.(a) Silicon-based anode swelling in-situ rapidscreening system (RSS1400); (b) NCM111 and NCM622 cathode materials assembled into a button-type full battery, and monitoring the swelling thickness change during three cycles of charge and discharge, Among them, NCM622 exhibits an “M” type of swelling behavior.
3. Results Analysis
The NCM cathode electrode belongs to the α-NaFeO2 type crystal [1], and its specific crystal structure is shown in Figure 2, where the green is lithium-ion, the blue is transition element (TM) ions, and the red is oxygen ions. The layered units composed of oxygen ions and transition element ions are arranged longitudinally along the c-axis, while lithium-ion are alternately distributed between these layered units along the c-axis, forming a typical ABC-type stacked cubic stacking structure [1]. F.B. Spingler et al. [2] studied the swelling of NCM cathode materials with different Ni contents and NCA cathode materials. The results are shown in Figure 3. It can be seen from Figure 3(a) that as the delithiation degree of the NCM111 cathode electrode deepens, its swelling curve is relatively flat at first, and even has a slight decline, and then presents a clear upward trend; when discharging lithium intercalation, the swelling curve also shrinks obviously first, and then tends to be gentle. With the increase of the Ni content of the cathode electrode, it can be found that the swelling of the cathodeelectrode decreases when the lithium is removed, and even shrinks at the end of the lithium removal (high voltage region), and this phenomenon is reversible when the lithium is inserted into the discharge. The details are shown in Figure 3(c) and (d).
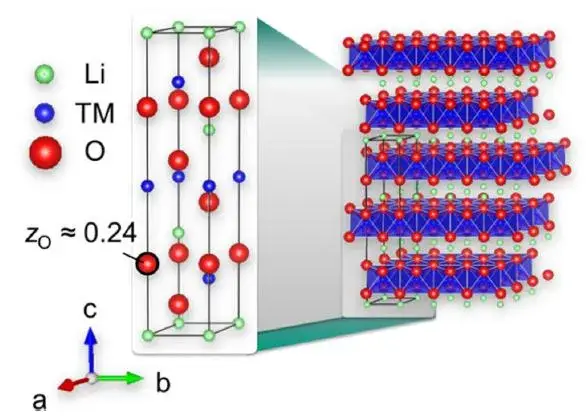
Figure 2. Schematic diagram of the crystal structure of layered LiNixCoyMnzO2[1].
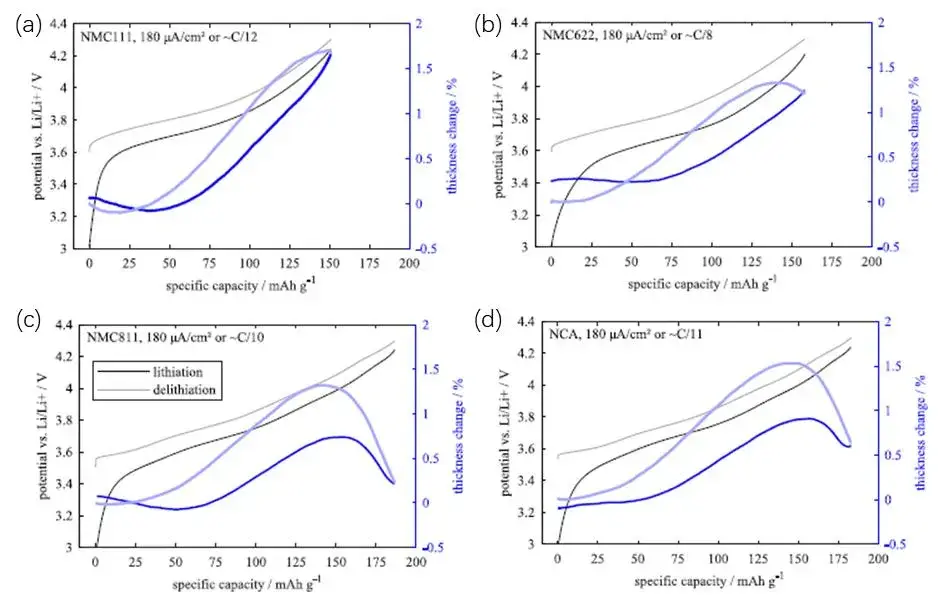
Figure 3. Variation of the swelling thickness of the ternary cathode with the gram capacity[2] (The upper limit cut-off voltage is 4.3V), where (a) is NCM111, (b) is NCM622, (c) is NCM811, (d) is NCA cathode.
To explain this special swelling behavior related to Ni content, L.D. Biasi et al[1] used in-situ XRD to study the angle change of the 003 crystal plane of ternary cathode materials with different Ni contents (NCM111, NCM523, NCM622, NCM721, NCM811 and NCM851005) with charging and delithiation, and the results are shown in Figure 4. With the increase of Ni content, the 003 crystal plane shifts to a higher angle direction under high voltage, indicating that the spacing of 003 crystal planes shrinks significantly under high voltage. Then, L.D. Biasi et al[1] analyzed the variation of the a-axis and c-axis of the NCM crystal with the voltage spacing, and the results are shown in Figure 5. When charging and delithiation, the a-axis will shrink first and then become flat; the c-axis will expand obviously first, and then start to shrink, and with the increase of Ni content, the degree of shrinkage of the c-axis in the second half will become more obvious, and the transition voltage from swelling to contraction will be significantly earlier. It is generally believed that the smaller spacing of the a-axis is related to the oxidation of the transition metal (TM), while the larger spacing of the c-axis is related to the increased Coulomb repulsion between the NCM crystal layers after the Li-ions are extracted, as the degree of delithiation deepens, the c-axis will generate a large number of voids (especially for high-Ni ternary materials), and eventually lead to structural shrinkage (that is, the spacing becomes smaller at high voltage). F.B. Spingler et al. [2] believe that microscopic swelling will accumulate and cause macroscopic reversible swelling, but microscopic shrinkage does not necessarily lead to macroscopic shrinkage, but will increase a certain gap in the electrode structure, the swelling and contraction of the c-axis are the main reasons for the swelling and contraction of the ternary material during charging, and as the Ni content increases, the shrinkage voltage of the c-axis will advance from 4.2V (vs. Li) to 4.0V (vs. Li), in the ternary-to-graphite full battery system, the voltage range is generally 3~4.2V, so we will find that for the low-Ni ternary full battery, it expands monotonically during charging and shrinks monotonically during discharge, for the high-Ni ternary full battery, it will first expand and then shrink when charging, and it will expand first and then shrink when discharging, showing an “M”-shaped swelling behavior.
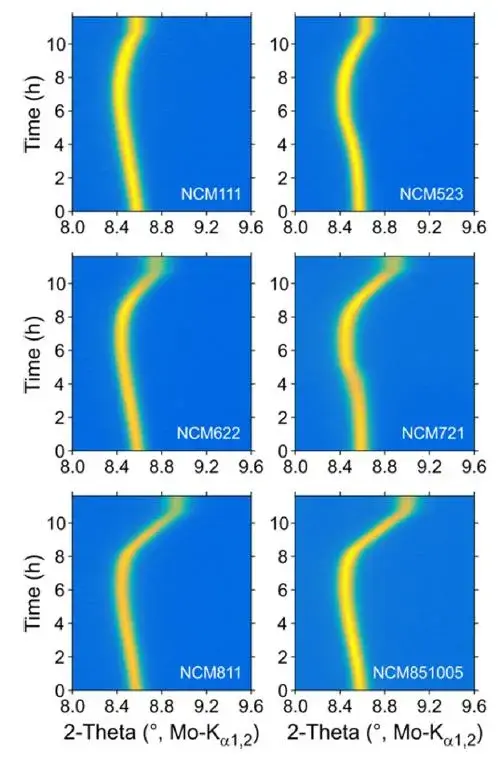
Figure 4. The change of the 2θ angle of the 003 crystal plane during charging of NCM cathode cells with different Ni contents under in-situ XRD test [1].
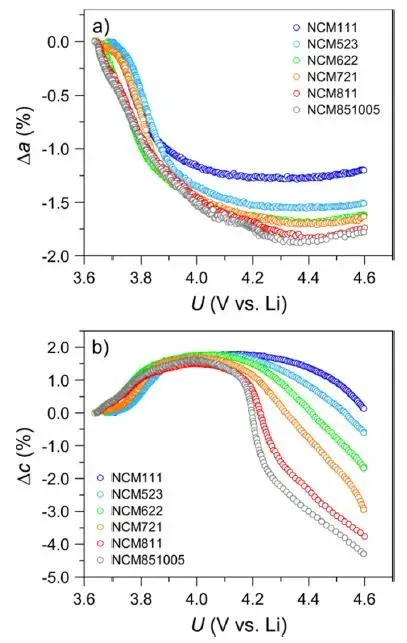
Figure 5. The relative spacing of the a-axis and c-axis with voltage during the charging and delithiation process of NCM cathode cells with different Ni contents [1].
We know that lithium cobalt oxide (LCO) also belongs to the α-NaFeO2 type crystal. B. Rieger et al. [3] also used in-situ XRD combined with the swelling test system to study the swelling behavior of the LCO cathode electrode during charging. The results are shown in Figure 6 shown. Similarly, although the a-axis shrinks during charging and delithiation (as shown in Figure 6(a)), the c-axis exhibits an obvious swelling behavior due to the increase of Coulomb repulsion, and finally leads to the macroscopic swelling of the LCO crystal. In the whole voltage range, the cathode electrode of LCO exhibits a monotonous swelling trend, and there is no volume shrinkage under high voltage, which is due to the absence of Ni element in LCO, the swelling behavior is consistent with that of low Ni ternary cathode materials.
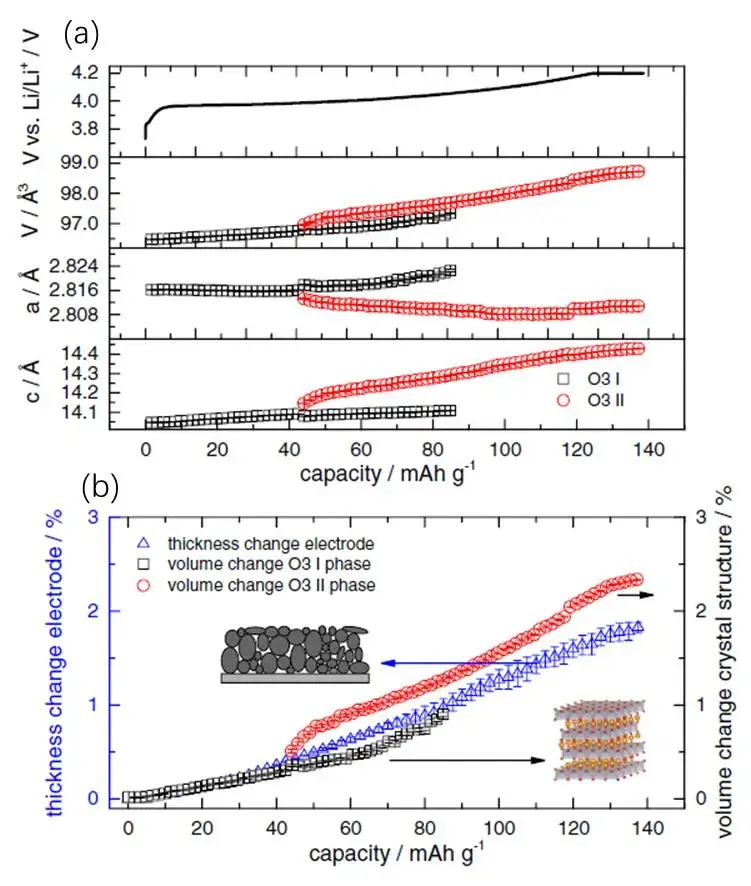
Figure 6. (a) When the O3 I phase changes to the O3 II phase, the a-axis, c-axis and unit cell volume change with the charging capacity; (b) During the charging process, the volume of the O3 I phase, the volume of the O3 II phase, and the thickness of the electrode sheet vary with the charging capacity.
4. Summary
In this paper, IEST‘s silicon-based anode swelling in-situ rapid screening system (RSS1400) was used to conduct swelling tests on the electrodes of the ternary system, and it was found that the high-Ni ternary system had an “M” type swelling behavior at high voltage, this is mainly caused by the special swelling behavior of high-Ni ternary cathode materials. According to literature analysis, whether it is LCO cathode electrode or NCM cathode electrode, the macroscopic structure will expand due to the increase of the Coulomb repulsion of the c-axis when charging and delithiation. When the Ni content in the ternary cathode materials is high, the charging swelling behavior will be transformed into a contraction behavior under high voltage. This is because after the delithiation degree is intensified, there are more voids in the c-axis, which will lead to the overall structure shrinkage. And the breakover voltage of this shrinkage behavior will advance with the increase of Ni content, so in the charge and discharge voltage range of 3~4.2V, the structure of high Ni ternary full battery will shrink at the end of charge, and reversible structural swelling occurs at the initial stage of discharge, finally showing an “M” type swelling curve.
5. References
[1] L.D. Biasi, A.O. Kondrakov, H. Gebwein, T. Brezesinski, P. Hartmann and J. Janek, Between Scylla and Charybdis: Balancing Among Structural Stability and Energy Density of Layered NCM Cathode Materials for Advanced Lithium-ion Batteries. J. Phys. Chem. C 121 (2017) 26163–26171.
[2] F.B. Spingler, S. Kucher, R. Phillips, E. Moyassari and A. Jossen, Electrochemically Stable In Situ Dilatometry of NCM, NCA and Graphite Electrodes for Lithium-ion Cells Compared to XRD Measurements. J. Electrochem. Soc. 168 (2021) 040515.
[3] B. Rieger, S. Schlueter, S.V. Erhard and A. Jossen, Strain Propagation in Lithium-ion Batteries from the Crystal Structure to the Electrode Level. J. Electrochem. Soc. 163 (2016) A1595-A1606.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


