-
iestinstrument
Study on the Correlation of Slurry Resistivity and Formula
1. Preface
Measure the slurry resistivity can assess the dispersion and conductivity of the particles at the slurry level. At present, conductive agent studies the model of conductive network, but quantitative analysis of resistivity is rare. This paper analyzes the relationship between slurry resistivity, formula, and viscosity, and verifies the conductivity type of anode and cathode electrode by changing the current-voltage curve.
Lithium anode and cathode electrode slurry is a solid-liquid two-phase mixed system formed by active substances, conductive agents and binders dispersed in solvent. Ideal electrode slurry should meet the following requirements: (1) active substances and conductive agent particles agglomerates as far as possible dispersion; (2) open the long chain of conductive agent, and further dispersion of chained conductive agent; (3) the formation of the most appropriate active substances, conductive agents and binders with each other’s arrangement; (4) to maintain the optimal suspension structure of the slurry and stability of the composition, to prevent the settling and agglomeration of components, such as precipitation and segregation. Among them, the homogeneity and stability of the slurry greatly affects the consistency and electrochemical performance of the core. If the solid particles are unevenly dispersed in the solvent or settle rapidly, a good electronic conductive network cannot be formed, which will greatly affect its electrical performance.
In the design of lithium-ion battery electrodes, the three-dimensional network formed by the conductive agent connects the active particles, which is the main path for electron transfer. Moreover, when the electronic conductivity of the active material, especially the cathode material, is very low, the conductive agent is also needed to promote electron conduction. Therefore, when designing Li-ion batteries, we should select the conductive agent that matches different active materials and different purposes (improving multiplication performance, cycling performance, and increasing irreversible specific capacity). Conductive agent materials, morphology, particle size, mixing order, the amount of addition and the composite state of different types of conductive agents have different aspects of lithium-ion batteries. In addition, the distribution state of the conductive agent is also very critical, the conductive agent in the slurry may be distributed as shown in Figure 1: (1) the conductive agent agglomerates together, not dispersed; (2) the conductive agent is uniformly dispersed, but individually suspended in the slurry, not and the active material conductive agent tightly combined; (3) the conductive agent in the slurry in the ideal distribution state: the conductive agent is uniformly dispersed in the conductive thin layer on the surface of the active material particles; Conductive agent and active material particles in close contact with the surface, so that electrons can effectively participate in the de/embedded lithium reaction; conductive agent interconnected conductive, and each active material particles to form an electronic pathway.
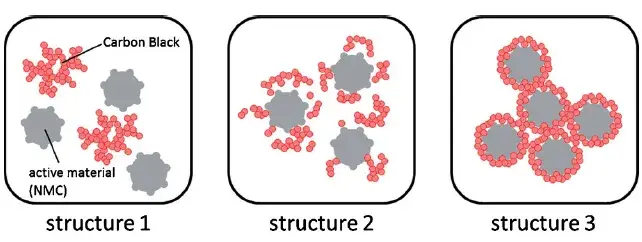
Figure 1. Distribution status of the conductive agent in the slurry
2. Test Method
2.1 Test Equipment
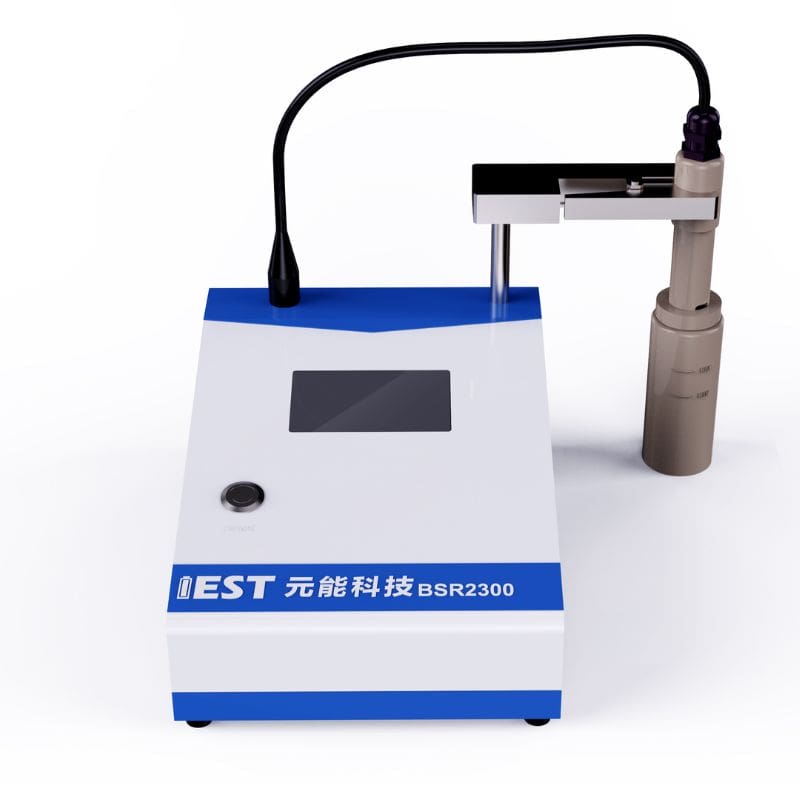
The IEST Lithium Battery Slurry Resistivity Tester(BSR2300) is used to represent the anode and cathode electrode slurry resistivity with different solid content and viscosity.
Figure 2. BSR2300 Appearance Diagram
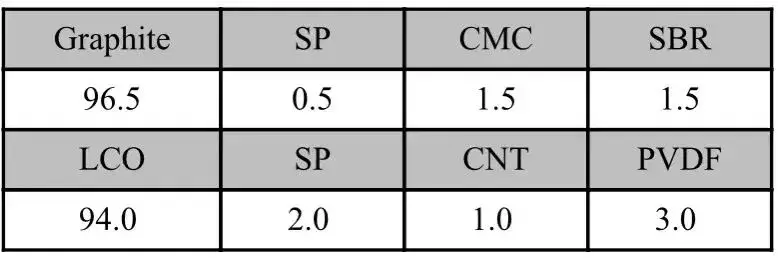
2.2 Slurry Ratio
Table 1. Slurry ratios
3. Test Results
According to the formula in Table 1, the anode and cathode electrode slurries with different solid contents were configured and tested for viscosity and resistivity, respectively, as shown in Table 2, both anode and cathode electrode slurries showed increasing viscosity and decreasing resistivity with the increase of solid content.
When the solid content of LCO is greater than 50%, the viscosity increases sharply, which may be due to the increasing proportion of lithium cobaltate particles per unit volume as the solid content increases, and the collision of large-density lithium cobaltate particles exacerbates the interaction force between the particles in the system, which leads to an increase in the viscosity, and the point of sharp increase is the threshold point of the sharp change in the force between the particles. When the graphite solid content of 35% or less, with the increase of solid content resistivity accelerated decrease, when the solid content is greater than 35%, the slurry resistivity with the solid content change is slower, this is because as the solid content increases, more and more particles to build an effective conductive network, when the conductivity is reached after the threshold is not a significant increase in conductivity.
Table 2. Anode and Cathode Electrode Slurry Resistivity & Solid Content & Viscosity
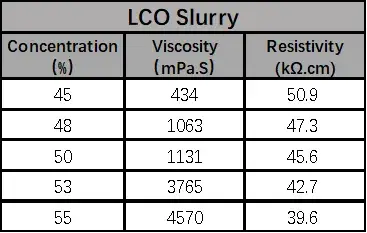
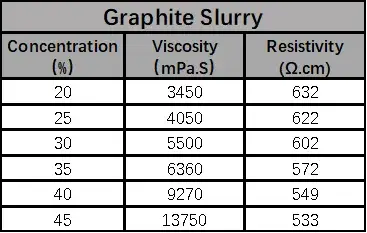
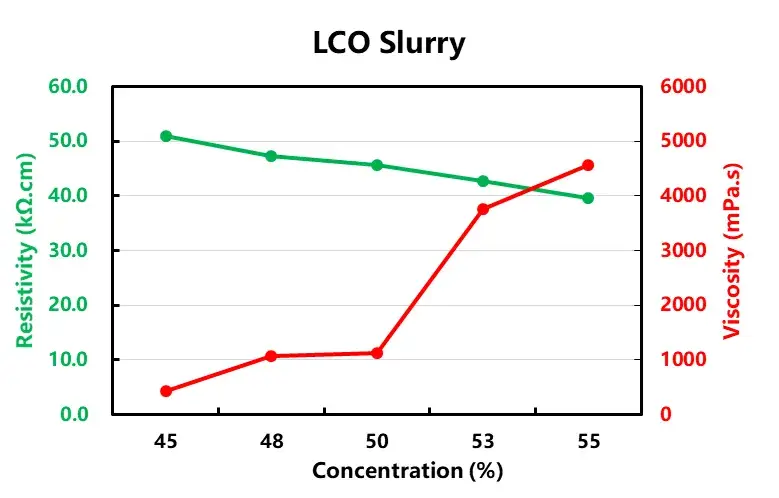
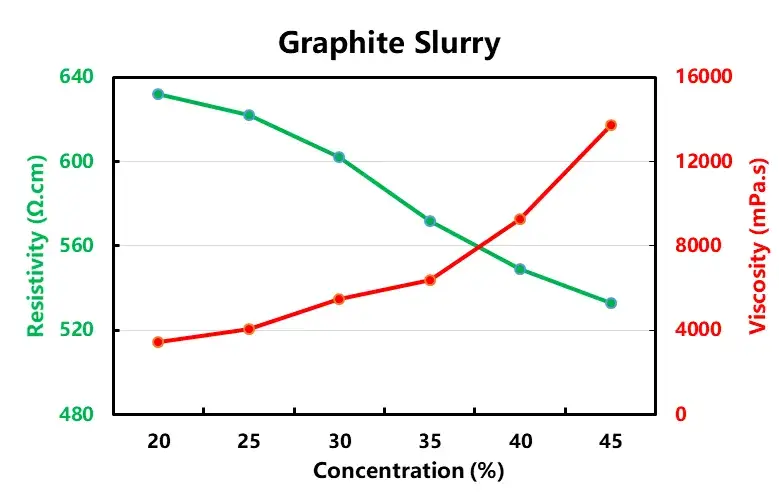
Figure 3. Trend of resistivity and viscosity of anode and cathode electrode slurry with solid content
In order to further investigate the conductive mode in the lithium positive and negative electrode slurry system, I-V experiments were designed to verify it, as shown in Figure 4. For LCO slurry and graphite slurry, 0.001mA, 0.0015mA, 0.002mA, 0.0025mA, 0.003mA current is applied respectively, and the voltage signal is collected. Figure 4 shows that the current-voltage linear relationship is obvious and basically composite Ohm’s law, indicating that the conductive type of lithium cobalt oxide cathode slurry and graphite anode slurry is mainly dominated by electron conductivity, i.e., the electrons are conducted to the particles themselves through the contact between the particles, which in turn builds a multidimensional conductive network and exhibits a certain degree of conductivity.
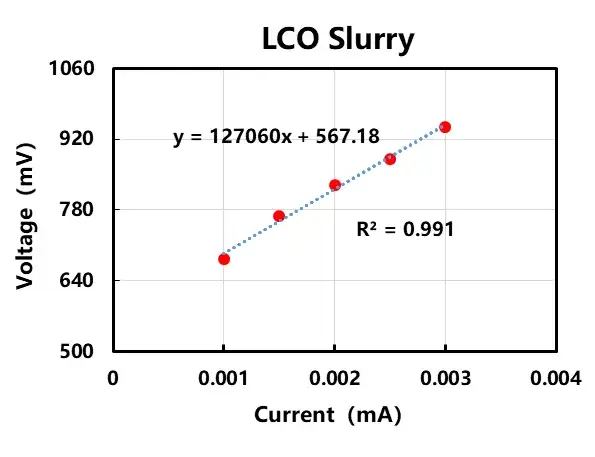
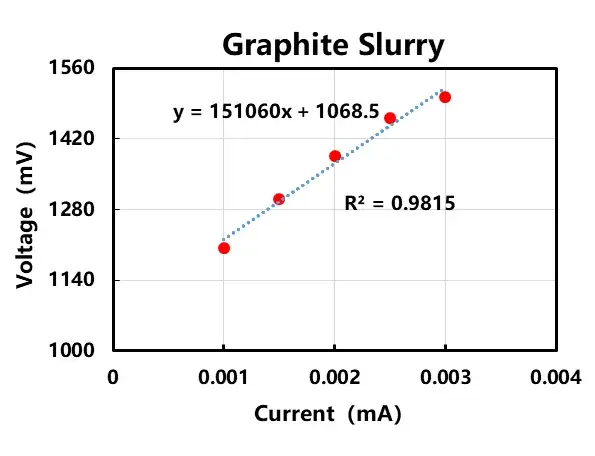
Figure 4. I-V curves for anode and cathode electrode slurry
4. Summary
In this paper, BSR2300 (IEST)was used to analyze the relationship between the slurry resistivity, viscosity and solid content of lithium battery anode and cathode electrode slurry, and found that the anode and cathode electrode slurry resistivity is significantly reduced with the increase of solid content. Meanwhile, through the I-V curve to prove that the lithium battery anode and cathode electrode slurry system is based on electronic conductance.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.