-
iestinstrument
Evaluation of Electrolyte Wetting Performance At The Electrode Level – Anode Electrode With Different Compaction Densities
1. Abstract
Electrolyte wetting is a critical process for lithium-ion cell performance: it controls ionic access to active material, influences rate capability and cycling, and affects initial manufacturing yield. This study uses a capillary-based electrolyte wetting test System (EWS1100) to quantify how varying compaction density of negative-electrode sheets alters electrolyte uptake kinetics and capacity for liquid uptake. Our results show a clear, essentially linear decline in electrolyte uptake with increasing compaction density, and demonstrate that the capillary method provides repeatable, discriminating data that traditional contact-angle or bulk immersion tests struggle to deliver.
2. Preface
The electrolyte is a core component in lithium-ion battery development, serving as the essential medium for ion transport and a foundation for achieving high voltage and specific energy. Its properties, particularly its electrolyte wetting behavior with electrodes and separators, directly impact overall battery performance. For electrodes, the wetting effectiveness is intrinsically linked to parameters like compaction density, pore size, and porosity. Quantifying electrolyte wetting at the electrode level is therefore a critical metric for process optimization and high-performance battery design. Similarly, the separator’s wetting characteristics are vital. Consequently, developing a reliable method to assess the electrolyte wetting of electrodes and separators is essential.
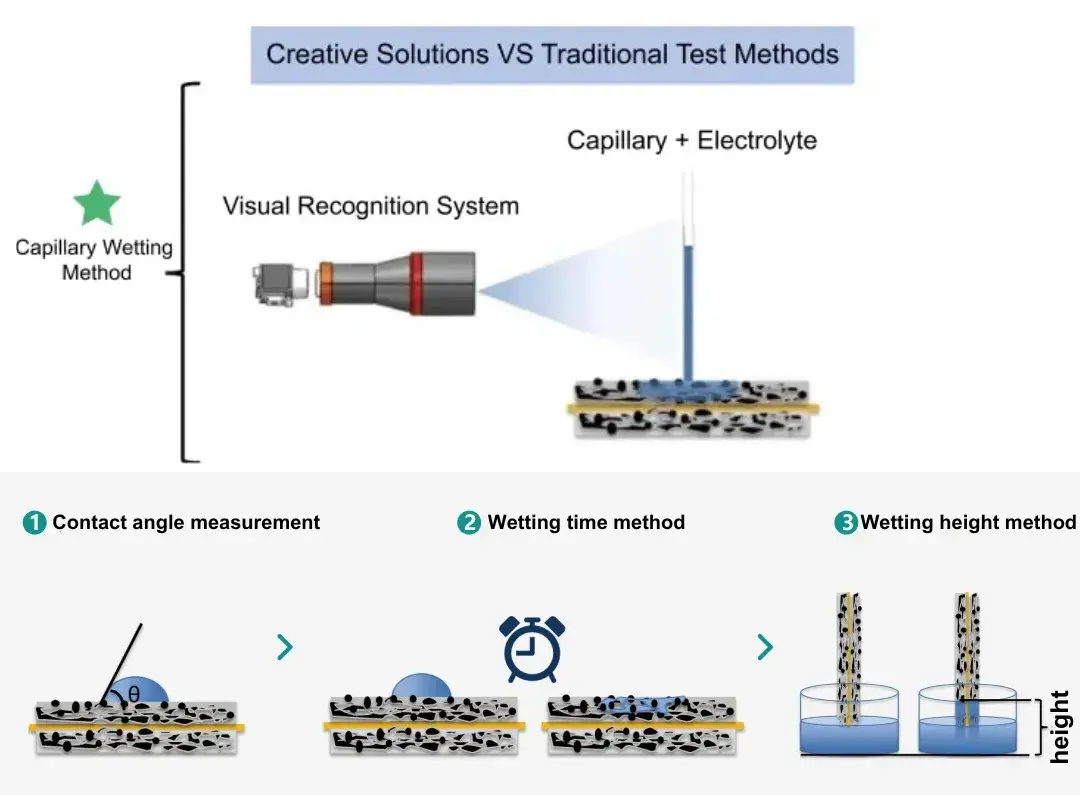
Figure 1. Schematic diagram of the capillary wetting method and traditional wetting test method
Traditional electrolyte wettability test methods generally include contact angle measurement method, wettability time method, wettability height method, etc., in which the contact angle measurement method is to add drops of electrolyte to the surface of the electrode or diaphragm, and determine the wettability of the electrolyte according to the contact angle of the electrolyte and the electrode or diaphragm, the electrolyte in the electrolyte or diaphragm spread on the surface of the test is faster, and the test is usually required to be equipped with a high-speed camera instrument. The overall test cost and difficulty are high, and it is difficult to examine the complete wetting time and wetting speed of the electrolyte on the electrode or diaphragm; the wetting time method is usually to take a certain amount of electrolyte drops on the surface of the electrode to test the length of time for the complete wetting of the electrolyte, and to evaluate the difference of electrolyte wetting of the electrode in terms of the difference in time; and the wetting height method is to completely immerse the fixed-size electrode in or one end of the electrode in electrolyte, and then determine the electrolyte wetting of the electrode in accordance with the time difference between the two. The immersion height method involves completely immersing a fixed-size electrode or immersing one end in electrolyte, and evaluating the electrolyte wetting performance of the electrode based on the mass of electrolyte immersed in the electrode within a certain period of time.
In order to solve the limitations of the traditional test methods, the R&D team of IEST has developed a set of Electrolyte Wetting Testing System(EWS1100), which can quantitatively evaluate the electrolyte wetting performance, based on the principle of capillary diffusion of the electrolyte in the electrode sheet and diaphragm, and the method of capillary wetting, equipped with a high-precision mechanical control system and a visual acquisition system, which can quantitatively evaluate the difference of electrolyte wetting between different positive and negative electrode sheets and diaphragms, and provide an effective means for electrolyte wetting evaluation. Figure 1 shows the schematic diagram of the principle of the capillary wetting method and the traditional wetting test method. The electrolyte is injected into the capillary tube, and after the capillary glass tube is vertically contacted with the surface of the electrode , the capillary liquid level decreases as the electrolyte continuously infiltrates the coating. The visual recognition system records the capillary liquid level height in real time, the dynamic evolution of the liquid level height is the electrolyte wetting process in real time, the height change is the amount of electrolyte wetting.
This paper is mainly based on the capillary wetting test system, combined with different compaction densities of anode electrode sheets for systematic testing, to evaluate the differences in electrolyte wetting of the electrode sheets at different compaction densities.
3. Methodology: A Novel Approach to Electrolyte Wetting Evaluation
3.1 Experimental Equipment
Model EWS1100 (IEST), the appearance of the equipment is shown in Figure 2.
Figure 2. Exterior view of the EWS1100 device
3.2 Sample Preparation and Testing
3.2.1 Sample Preparation
After uniformly coating the slurry under the same process formulation conditions, we respectively used different pressures, such as small, medium and large, for roll pressing, and obtained a total of four compacted finished anode electrode sheets, in which the pressure of the electrode sheets roll pressing was 1<2<3<4. The compaction density of the four electrode sheets was calculated by means of cutting-thickness-measuring-weighing, and the size of the compaction density was also presented as Sample 1 (1.35 g/cm³), Sample 2 (1.5 g/cm³), Sample 3 (1.6 g/cm³), and Sample 4 (1.65 g/cm³), with the increase of the roller pressure, the compaction density also shows an increasing trend.
3.2.2 Testing process
Pre-treatment of samples to be tested → sample standardization and fixation → equipment on-line and software parameter design → capillary automation suction → capillary automation downward pressure test → visual recognition system real-time monitoring of capillary liquid surface height → data acquisition and processing.
3.2.3 Electrolyte wetting test of different compaction densities electrodes
Combined with EWS1100 equipment, the capillary wettability test of 1/2/3/4 electrodes was conducted respectively to compare the wettability difference between different electrodes.
4. Results & Discussion: Linking Compaction Density to Electrolyte Wetting Performance
4.1 Quantifying the Inverse Relationship
The electrolyte wetting of the electrode is closely related to the porosity of the electrode, through the particle size and distribution of the material, particle morphology and compaction density can effectively adjust the porosity of the material and the distribution of the voids, which in turn directly affects its electrolyte wettability. Research shows that the higher the compaction density, the lower the porosity and the smaller the diameter of the most frequent voids.
Figure 3 shows the electrolyte wetting curves of four different compaction densities of the electrode, from the curve, with the increase of compaction density, the slope of the electrolyte wettability curve of the electrode decreases gradually, i.e., the greater the density of compaction, the worse the wettability, which is consistent with the reported studies;
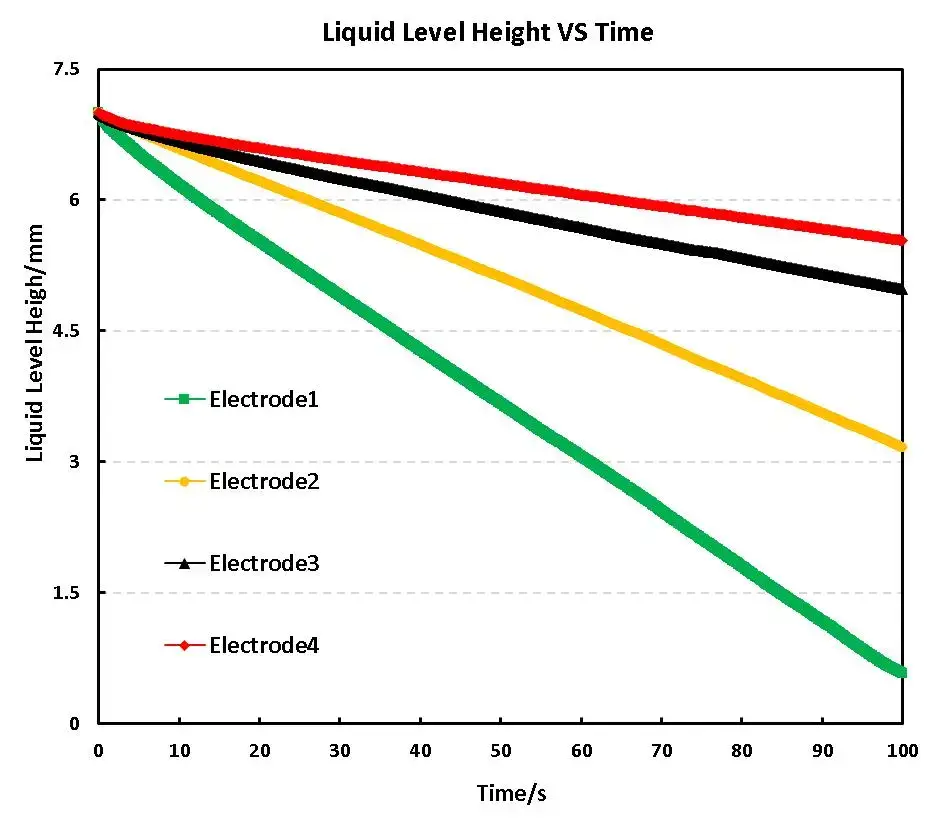
Figure 3. Electrolyte wetting curves for different compaction densities electrodes
Figure 4 shows the relationship between electrolyte wettability of the electrode and the change of the density of compaction at different points in time(50s, 60s, 100s), as can be seen in the figure, at each interval, the uptake amount decreases linearly with increasing compaction density. Notably, the slope of this linear relationship becomes steeper with longer wetting times, suggesting that the negative impact of high compaction density on wetting becomes more pronounced over time.
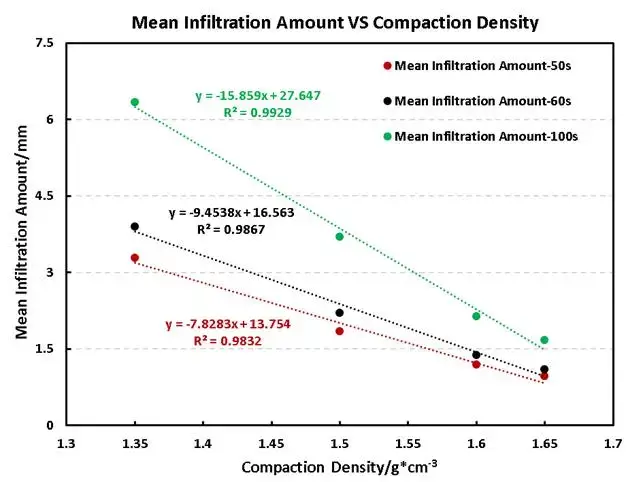
Figure 4. Variation curves of electrolyte wetting with compaction density for electrodes at different time points
4.2 Understanding the Dynamics with the Lucas-Washburn Model
The actual positive and negative electrodes are porous structures, which can also be regarded as capillary structures with different pores, and the wetting process of the electrolyte in the electrode can be understood as the capillary absorption effect. The Lucas – Washburn wetting model is usually used to describe the kinetics of liquid absorption by the capillary effect in the electrode sheet, as shown in Equation (1)-, where h is the liquid absorption height, t is the liquid absorption time, c is the shape coefficient corresponding to the capillary with different gaps, r is the capillary radius, cr is a constant value called the formal radius, σ is the surface tension of the liquid, and η is the liquid viscosity.
Washburn’s equation can better describe the relationship between conduction distance and conduction time when the liquid conducts horizontally and vertically, combining the results of Fig. 3 & Fig. 4, the results confirm that wetting involves both vertical imbibition and horizontal diffusion. For thin electrodes where thickness is much smaller than length and width, the process can be considered primarily radial. Electrodes with different compaction densities have different porosities and different amounts of electrolyte that can be absorbed, and the effect of compaction density becomes more significant as the wetting time increases.
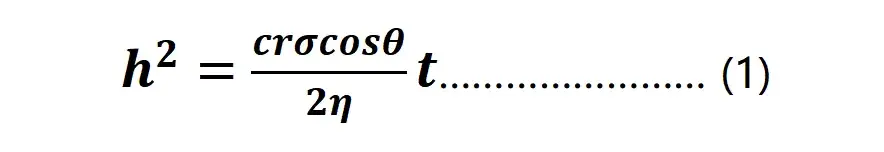
4.3 Assessing Test Repeatability and Accuracy
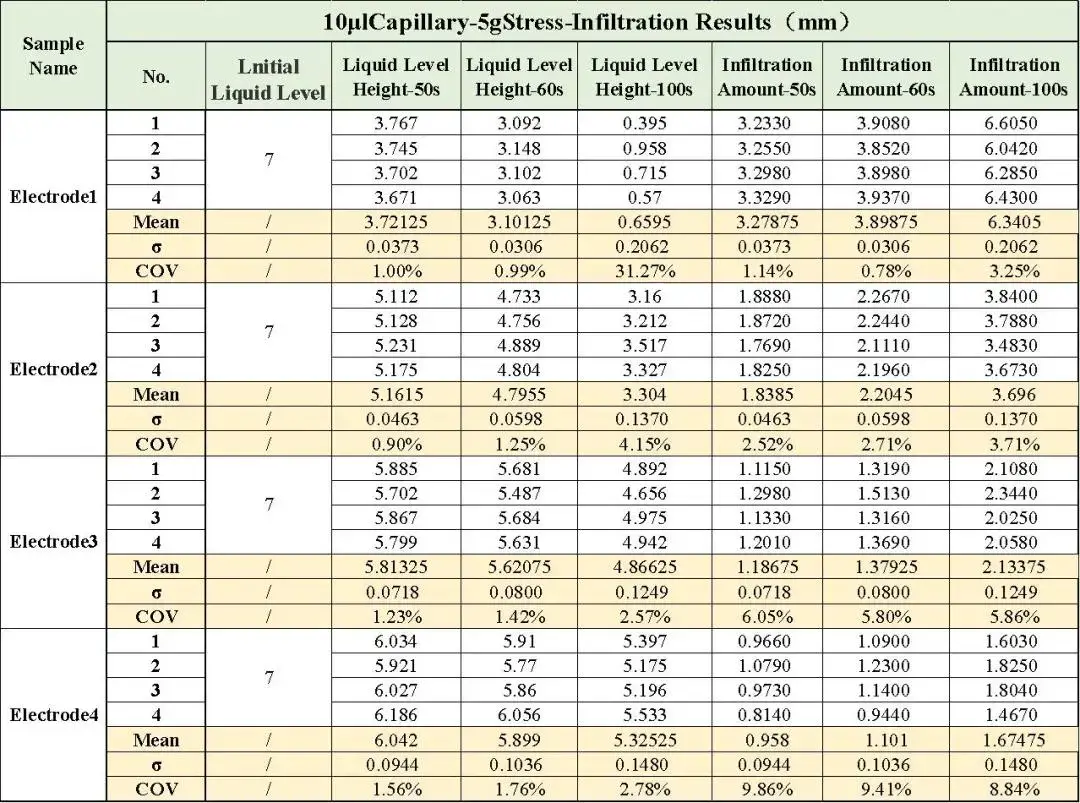
Table 1 is a summary of the electrolyte wetting repeatability test of different compacted electrodes at different times, where each sample was tested in 4 groups for repeatability, and the mean, standard deviation and coefficient of variation were calculated, show standard deviations below 0.15 and Coefficients of Variation (COV) controlled within 10% for all samples. This confirms the method’s accuracy and reliability for distinguishing electrolyte wetting performance.
Table 1. Summary of electrolyte wetting results of different electrodes at different times
5. Practical implications for electrode design and manufacturing
Several immediate implications follow:
-
Balance energy density and wetting: Increasing compaction density raises volumetric active material loading and volumetric capacity, but it also impairs electrolyte wetting and ion transport. Design choices must therefore balance volumetric energy gains against potential penalties in rate capability and cycle life.
-
Process optimization: Because wetting becomes more rate-limited at higher compaction density and over longer times the compaction parameters (calender pressure and resulting thickness) should be optimized together with electrolyte formulation (viscosity, surface tension) and upstream particle morphology to achieve fast, complete wetting without sacrificing areal or volumetric capacity.
-
Measurement utility: The capillary method implemented on EWS1100 shows sufficient sensitivity and repeatability to be used as a screening tool during electrode process development, enabling rapid comparisons of formulation or calendering changes.
6. Summary
This study successfully employed the innovative capillary wetting method(EWS1100) to evaluate the electrolyte wetting performance of anode electrodes with varying compaction densities. The system effectively distinguished wetting differences between samples, with results aligning perfectly with known principles—higher density leads to poorer wetting. The method overcomes limitations of traditional contact-angle or bulk immersion tests, offering a repeatable, discriminating means to evaluate electrolyte migration at the electrode-sheet level. It serves as a powerful tool for optimizing both electrolyte formulation and electrode microstructure design, ultimately aiming for rapid, complete wetting to reduce production costs and enhance battery quality.
7. References
[1] Yang Shaobin, Liang Zheng. Lithium-ion Battery Manufacturing Process Principles and Applications.
[2] PFLEGING W, PROLL J. A new method for rapid wetting of electrolyte for lithium-ion battery tape-cast electrodes[J]. Journal of Materials Chemistry A, 2014, 2: 14918-14926.
[3] SCHILLING A, GUMBEL P, MOLLER M, et al. X-ray Based Visualization of the Electrolyte Filling Process of Lithium Ion Batteries[J]. Journal of the Electrochemical Society,2019,166:A5163-A5167
[4] Edward W. Washburn. The Dynamics of Capillary Flow[J]. Physical Review, 1921, 17: 273-283.
[5] LI K W, ZHANG D F, BIAN H Y, et al. Criteria for applying the Lucas-Washburn law[J]. Scientific Reports, 2015, 5: 14085.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



