-
iestinstrument
Energy of Activation Calculation And Temperature Changing Conductivity Testing Calculation Of Anode And Cathode Electrode Materials And Solid Electrolytes
1. Preface
Energy of activation (Ea) is commonly used to define the energy barrier that needs to be overcome for a chemical reaction to occur. The energy required for a molecule to change from a normal state to an active state in which a chemical reaction can easily take place is called energy of activation , and this concept was proposed by S.A. Arrhenius of Sweden in 1889 on the basis of summarizing a large number of experimental facts and obtaining an empirical formula:
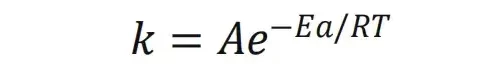
For a primary reaction, the energy of activation can be used to express the minimum energy required for a chemical reaction to occur, the magnitude of which can reflect the ease with which a chemical reaction can occur, as well as the energy required for a crystal atom to migrate out of its equilibrium position to another new equilibrium or non-equilibrium position. For example, the energy that needs to be overcome in order to start a physicochemical process (e.g. plastic flow, electron/ion diffusion, chemical reaction, hole formation, etc.). This energy can be provided by the energy fluctuations of the system itself or from outside sources. The smaller the energy of activation, the easier the process is to carry out.
Temperature characterization of lithium-ion batteries is a very important part of battery technology research. Temperature has a significant impact on the performance and lifetime of lithium-ion batteries, so the study of the temperature characteristics of lithium batteries is essential to achieve efficient, safe, and long-lasting battery operation. The temperature characteristics of a battery are the result of the combined effect of multiple components of the materials inside the battery (e.g., cathode, anode, diaphragm, and electrolyte, etc.). However, the lithium-ion battery as a whole for the systematic evaluation of the temperature characteristics of the test can only get the regularity of the test, can not be from the principle of its analysis and subsequent improvement; therefore, the separate testing of the temperature characteristics of different components of the material, and to establish a link between the different components, that is, in-depth understanding and analysis of the temperature characteristics of lithium-ion batteries is the necessary way, and for the temperature characteristics of the optimization of the improvement of providing an effective means and data support. It is a necessary way to deeply understand and analyze the temperature characteristics of Li-ion batteries, and provides an effective means and data support for optimizing and improving the temperature characteristics.
Therefore, the establishment of effective testing and characterization means to study the temperature characteristics of different component materials of lithium-ion batteries, and combined with the relevant theory of activation energy, the temperature characteristics of lithium-ion battery-related materials can be analyzed and improved from the principle; at the same time, it also provides the relevant theoretical calculations of the research and development personnel with the reliable data support required for simulation calculations.
2. Experimental Equipment and Testing Methods
In lithium-ion batteries, the electrodes are a mixed conductor of electrons and ions (solid particles of the active material and conductive agent conduct electrons, and the electrolyte conducts ions), while the separator or solid electrolyte is mainly an ion conductor. In this article, the Powder Resistivity & Compaction Density Tester PRCD3100 independently developed by IEST is used. This device is equipped with a newly developed temperature-raising device to test the electronic conductivity of different materials at different temperatures. In addition, with the testing system independently developed by IEST for solid electrolytes, solid electrolyte sheets can be pressed continuously and stably. With an external electrochemical workstation, the ionic conductivity of solid electrolytes at different temperatures can be tested in-situ.
Figure 1. (a) PRCD3100; (b) temperature increasing device; (c) solid electrolyte testing system
3. Results Analysis
The powder resistivity test of lithium iron phosphate (LFP) material was carried out at different temperatures in the pressure range of 10-200 MPa, as shown in Fig. 2(a), the resistivity decreases with increasing temperature at different pressures. And the trends of resistivity with increasing pressure at different temperatures are similar. Combined with the Arrhenius formula for analysis, we can take the logarithm of the Arrhenius formula to get:
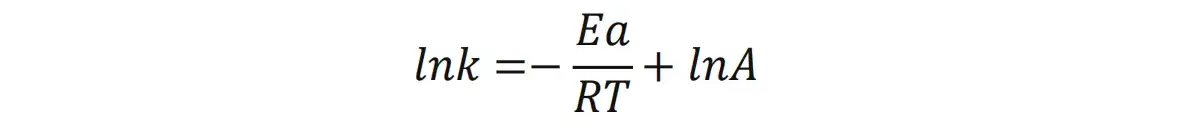
Bring the rate coefficient k in the formula into the conductivity and get the relationship between conductivity and temperature. By testing the conductivity of the material at different temperatures, the slope and intercept can respectively correspond to the energy of activation (Ea) and pre-exponential factor (A) after linear fitting.
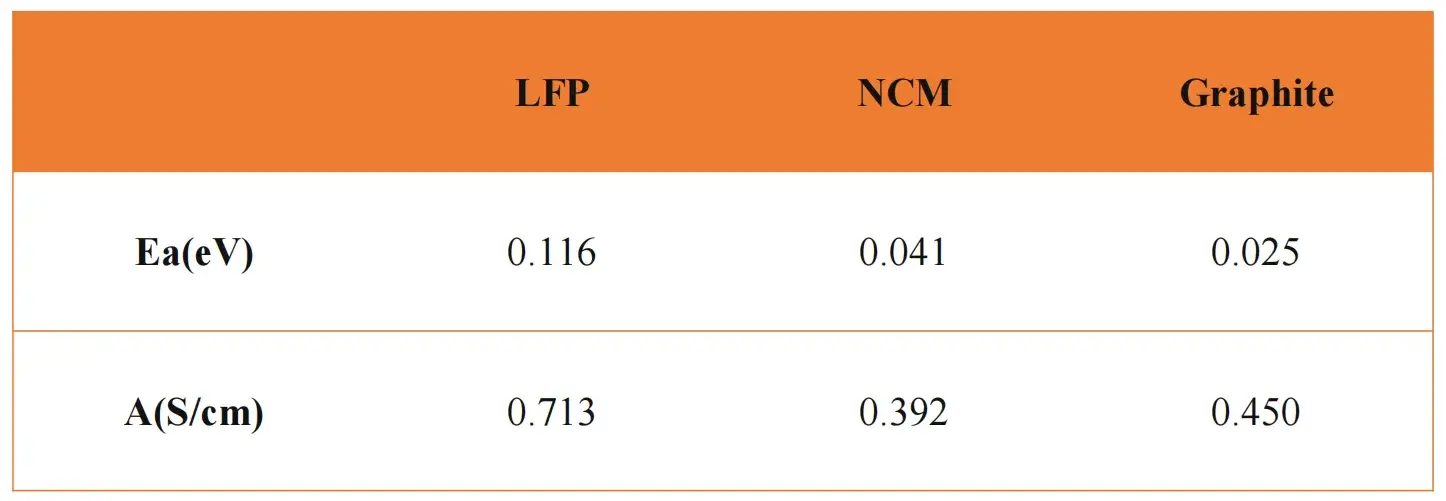
Select powder resistivity data at different temperatures under the same pressure, calculate the conductivity, and then combine the Arrhenius formula to make the corresponding linear fitting curve of lnσ and 1/T. Further calculations can obtain the corresponding energy of activation (Ea). As shown in Figure 2 (b), in addition to LFP, we also tested the electrical conductivity of ternary materials (NCM) and graphite (Graphite) at different temperatures, according to the Arrhenius formula, the energy of activation and pre-exponential factor were calculated separately. The calculated results are shown in Table 1. From the comparison of energy of activation indicators, lithium iron phosphate has the largest energy of activation, about 0.116eV; the energy of activation of ternary materials is slightly smaller than lithium iron phosphate, about 0.041eV; the energy of activation of graphite materials is the smallest, about 0.025eV. The above results show that among the three materials, the energy that electrons need to overcome for transmission in graphite material is the smallest, followed by the ternary material, and the energy that needs to be overcome for transmission in lithium iron phosphate material is the largest.
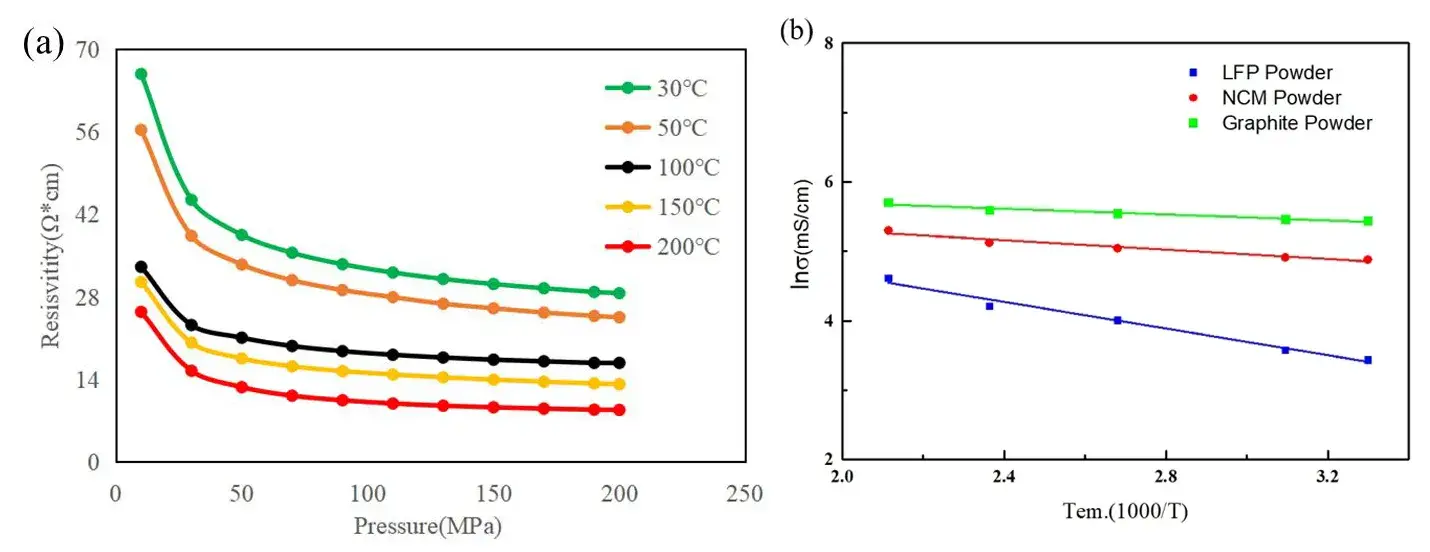
Figure 2. (a) Resistivity of LFP powder between 10 and 200MPa at different temperatures;
(b) Arrhenius plot of conductivity versus temperature of different positive and negative electrode materials.
The electronic conductivity of battery electrodes is one of the key factors that determine the performance of lithium-ion batteries. Typically, an electrode sheet contains active material, conductive carbon, and a binder. In the current research, the impact of the type and proportion of the conductive agent in the electrode piece on the electronic conductivity of the electrode piece is mainly considered. Especially for the positive electrode, since the electronic conductivity of the active material is very low, conductive additives are used to ensure good electronic conductivity. However, in high-energy batteries, the levels of conductive carbon and binder need to be as small as possible. In conductive and insulating composite materials, electronic conductivity is often explained based on permeation theory, with the conductive agent considered to be the conductor and the other components (i.e., active materials, binders, and pores) considered to be insulators. However, the electrode density and the mass ratio of carbon black have different effects on electrical conductivity. In addition to conductive carbon, the type and volume fraction of active materials also have an impact on electrical conductivity. Therefore, the impact of the electronic conductivity of the active material itself on battery performance should also be taken into consideration. Our testing methods and data this time have a certain reference value for studying the impact of electronic conductivity of active materials.
Table 1. Calculated results of energy of activation and pre-exponential factor of different positive and negative electrode materials
Solid electrolytes still face huge challenges to further improve their ionic conductivity to meet practical application requirements. Among them, the basic step of the lithium diffusion path is that Li ions migrate between two stable sites through a high-energy transition state. Reducing the transition state energy of activation of the long-distance diffusion path is of great significance to improving the ionic conductivity. Therefore, for solid electrolyte materials, we conducted electrochemical impedance spectroscopy (EIS) tests on oxide solid electrolyte LATP materials at different temperatures, as shown in Figure 3(a), the Nyquist diagram shows a curve with only the ion diffusion resistance part in the low frequency region. The curve shifts significantly to the left as the temperature increases, and the ion resistance decreases with the increase in temperature.
Calculate the ionic conductivity of LATP at different temperatures, and combine the Arrhenius formula to make the corresponding linear fitting curve of lnσ and 1/T. The corresponding energy of activation can be obtained through further calculations. As shown in Figure 3 (b), after calculation, the energy of activation of the LATP sample is 0.044eV.
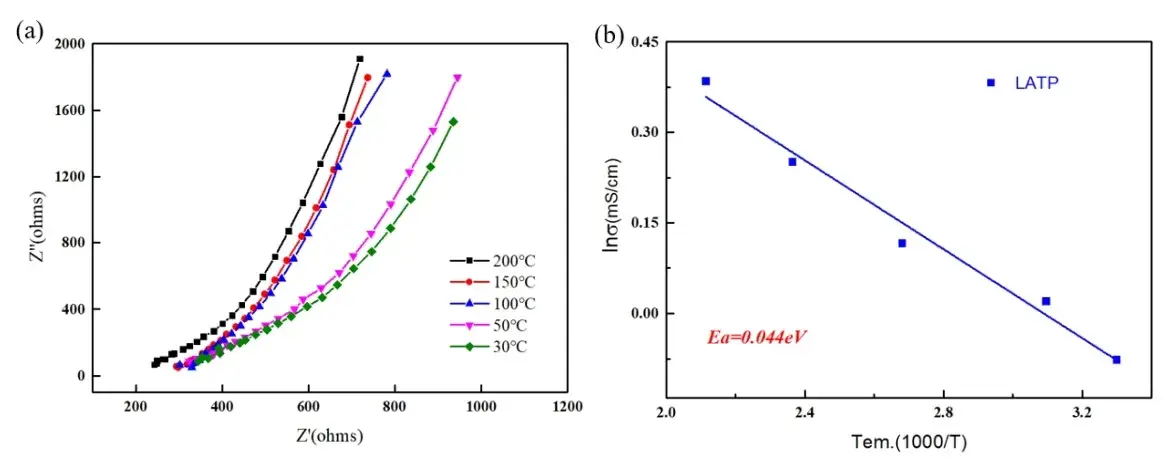
Figure 3. (a) Nyquist plots corresponding to Fig. 1(a) for LATP materials at different temperatures;
(b) Arrhenius plots of ionic conductivity versus temperature for LATP materials
During the test process of solid electrolyte ionic conductivity, on the one hand, the density, roughness and integrity of the pressed solid electrolyte sheet will affect the test results of the solid electrolyte conductivity; on the other hand, only stable and uniform force application during the test can ensure the accuracy of the test results. The testing system for solid electrolytes independently developed by IEST can continuously and stably press solid electrolyte tablets; at the same time, it can apply stable and standardized pressure, which plays an important role in the solid electrolyte and its lithium metal battery.
3. Summary
When exploring the temperature characteristics of material conductivity, the conductivity of the material tested at different temperatures can analyze the electron/ion transport capacity of the material at the current temperature point. Combined with the energy of activation results, the change in the intrinsic temperature properties of the material can be clarified, providing an effective means of analysis for basic materials and engineering research, as well as the data support required for simulation calculations by relevant theoretical calculations developers. The finger front factor (A) is a parameter determined by the intrinsic nature of the material, independent of temperature and material concentration, and it has the same magnitude as the property under study (e.g., electrical conductivity). The magnitude of the prefactor is also determined by the properties of the material itself, which is of some research significance, while its relevance needs to be explored by researchers.
4. References
[1] Wu Wenwei. Concise Inorganic Chemistry[M]. Chemical Industry Press, 2019.
[2] Weng S, Zhang X, Yang G, et al. Temperature-dependent interphase formation and Li+ transport in lithium metal batteries[J]. Nature communications, 2023, 14(1): 4474.
[3] Zhao Q, Liu X, Zheng J, et al. Designing electrolytes with polymerlike glass-forming properties and fast ion transport at low temperatures[J]. Proceedings of the National Academy of Sciences, 2020, 117(42): 26053-26060.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.