-
iestinstrument
Entering Electrochemistry | GITT Analyzes The Diffusion Kinetics Of Lithium Batteries
1. Preface
The Galvanostatic Intermittent Titration Technique (GITT) is a widely used electrochemical measurement method to evaluate the lithium-ion diffusion coefficient in battery electrode materials. As a core GITT battery characterization tool, it provides insights into how lithium ions move through electrodes by analyzing the relationship between voltage and time during intermittent current pulses.[1] [2]
A lithium-ion battery operates as a “rocking-chair” system, where lithium ions shuttle between the cathode and anode through the electrolyte (Figure 1). During charging, lithium ions migrate from the cathode to the anode, while electrons flow through the external circuit. In discharging, the opposite occurs, generating usable electric power. Since lithium-ion transport directly impacts charging/discharging efficiency, cycle life, and temperature performance, accurate GITT test analysis of ion diffusion is essential for battery optimization.
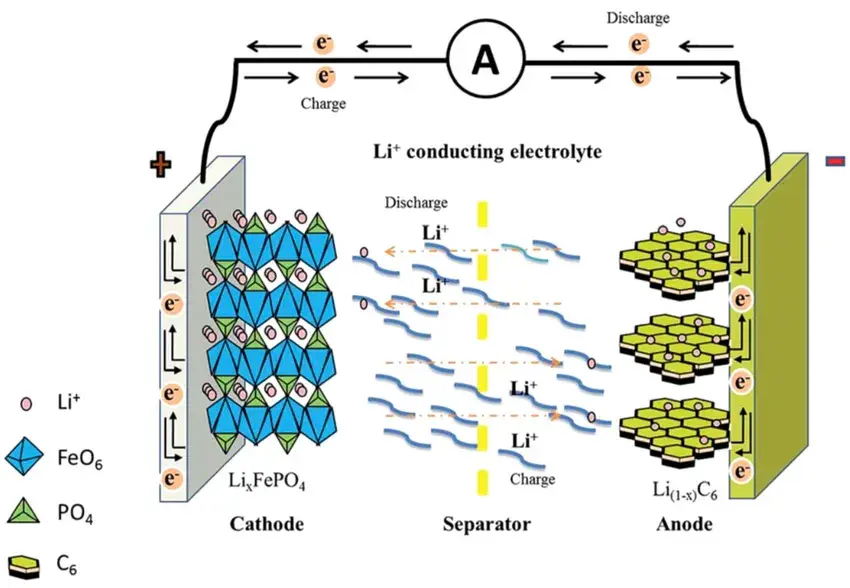
Figure 1. Schematic diagram of the working principle of lithium-ion battery
2. Basic Overview of the GITT Test
The overall GITT process consists of a series of “pulse-constant current-relaxation” processes (Figure 2). A set of “pulse-constant current-relaxation” process is to charge/discharge the battery by applying a constant current for a certain period of time, and then disconnecting the current and recording the voltage change of the whole process, the key of the test is the constant current and the accurate voltage. In the relaxation phase after disconnecting the current, it is necessary to let the lithium ions diffuse fully inside the active material, and the diffusion coefficient is further calculated by the relationship between voltage and time. In order to satisfy the assumption of the GITT method that “the diffusion process mainly occurs in the surface layer of the solid-phase material”, it is necessary to make certain limitations on the test conditions:
Key conditions for GITT analysis:
-
The pulse time t must be short enough to satisfy the condition t << L²/D, where L is electrode thickness and D is diffusion coefficient.
-
The relaxation time must be long enough to allow lithium ions to reach equilibrium within the active material.
Through this approach, the GITT battery method effectively captures the voltage response linked to ion transport kinetics.
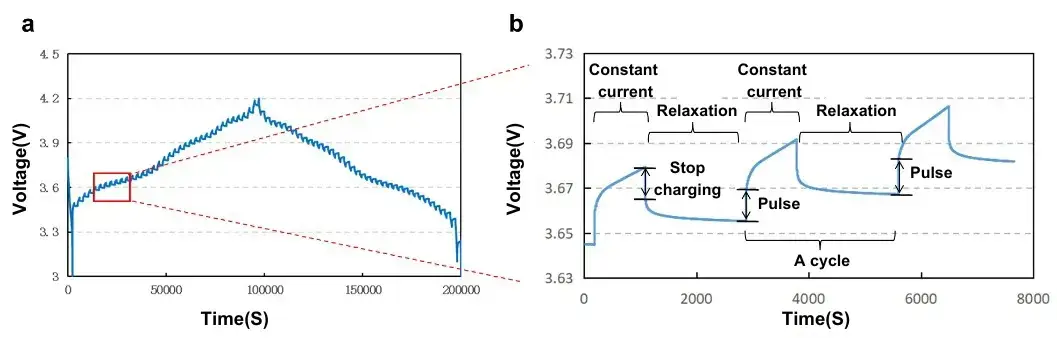
Figure 2. (a) GITT test curve and (b) localized zoomed-in schematic
3. Core Formula in GITT Analysis
The GITT test data allows for further calculation of the corresponding diffusion coefficients with the following equations:
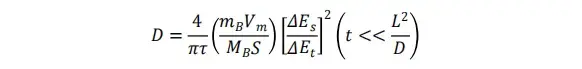
-
D = lithium-ion diffusion coefficient
-
mB = active material mass
-
Vm = molar volume of electrode
-
MB = relative molecular mass
-
S = electrode surface area
-
τ = relaxation time
-
ΔEt = voltage change during current pulse
-
ΔEs = voltage change during relaxation phase
-
t = pulse time
-
L = electrode thickness
By substituting ΔEs, ΔEt, and material constants into the equation, precise values for D are obtained.
In general, the voltage changes obtained during the test include not only the surface diffusion values, but also the voltage changes that reflect the SOC changes. However, the challenge in GITT analysis lies in ensuring accuracy: shorter pulse times improve theoretical precision but reduce the magnitude of ΔEs, requiring highly accurate instruments. For example, the IEST analyzer achieves 0.01% measurement accuracy across 8 testing channels, enabling reliable GITT battery characterization.
4. Application Cases
4.1 Lithium-ion Diffusion at Different SOC States
The authors investigated the changes of Li-ion diffusion coefficient during the charging and discharging process of LiNi0.8Co0.1Mn0.1O2(NCM811) by means of GITT test[3]. The value of lithium ion diffusion coefficient DLi+ varies significantly in different SOC states. The values of DLi+ were 10-8~10-9cm2 s-1 during charging and 10-7~10-11cm2 s-1 during discharging. At the beginning of charging, DLi+ increased with the release of lithium ions, reached a maximum value at a lithium content of~0.5, and then gradually decreased. The diffusion coefficient decreases rapidly when the lithium content is lower than 0.2. In addition, during the discharge process, DLi+ was extremely high at the beginning; after that, the value decreased slightly and remained at a high level with the insertion of lithium ions. When the Li-ion embedding content reaches 0.8, the DLi+ drops sharply by three orders of magnitude. This very low Li-ion embedding kinetics explains the capacity loss in the first cycle.
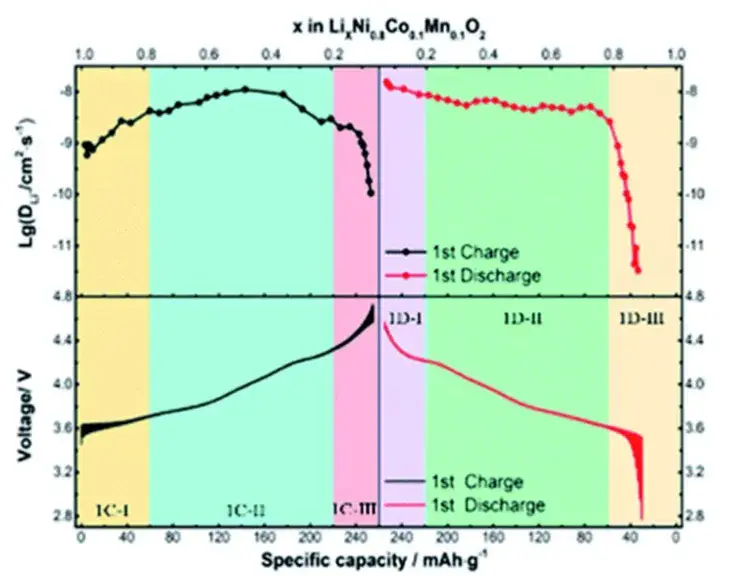
Figure 3. First turn GITT curve and Li ion diffusion coefficient of NCM811
4.2 Effect of material modification on ion diffusion coefficient
The authors introduced high entropy elements (Cr, Mn, Fe, Zn, Al) into the NASICON structure Na3V2(PO4)3 (NVP) to obtain NNa3V1.8(CrMnFeZnAl)0.2(PO4)3(HE-NVP-0.2) in order to realize the material’s crystal structure tuning and diffusion ability [4]. As shown in Figures 4a and 4b, the GITT battery study results show that the HE-NVP-0.2 electrode exhibits better Na ion diffusion kinetics after the introduction of high entropy elements. After NVP and HE-NVP-0.2 were assembled into half-cells and the rate performance was tested, it was found that the rate performance of HE-NVP-0.2 was significantly better than that of the NVP sample (Figure 4c).
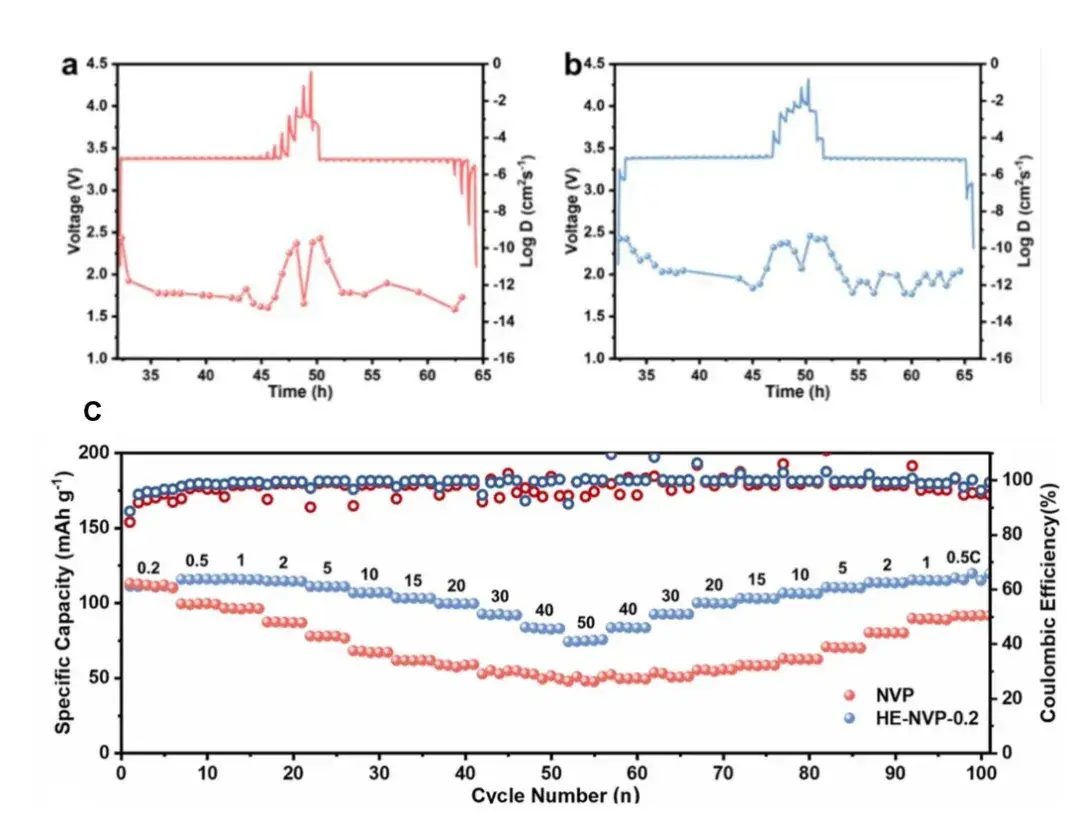
Figure 4. (a) GITT curves and corresponding Na ion diffusion coefficients for NVP and (b) HE-NVP-0.2
5. Summary
The diffusion behavior of lithium ions within the active material reflects the microscopic kinetic performance of the battery and greatly affects the overall performance of the battery. Segmented studies of electrochemical reactions at different charging and discharging depths can effectively find the key factors affecting the polarization of the battery at each stage, and GITT test can effectively determine the diffusion coefficient of lithium ions, D, and thus study the kinetic process of the battery.
Based on the importance of GITT analysis to the study of lithium-ion batteries, IEST has independently developed a high-precision electrochemical performance analyzer, which integrates the GITT test into the conventional charging and discharging equipment, templates the work steps, makes the setup simple, and makes the operation easy to improve the testing efficiency. The device also integrates CV (Cyclic Voltammetry), EIS (Electrochemical impedance spectroscopy) and LSV (linear scanning voltammetry) modules to enable R&D personnel to quickly conduct relevant electrochemical performance studies (Figure 5). In addition, the IEST electrochemical analyzer is equipped with advanced data processing and analysis software that enables real-time processing and multi-dimensional analysis of complex electrochemical data.
Figure 5. IEST electrochemical analyzer
6. References
[1] Nickol A, Schied T, Heubner C, et al. GITT analysis of lithium insertion cathodes for determining the lithium diffusion coefficient at low temperature: challenges and pitfalls[J]. Journal of The Electrochemical Society, 2020, 167(9): 090546.
[2] Tang K , Yu X , Sun J ,et al. Kinetic analysis on LiFePO4 thin films by CV, GITT, and EIS[J].Electrochimica Acta, 2011, 56(13):4869-4875.
[3] Hong C, Leng Q, Zhu J, et al. Revealing the correlation between structural evolution and Li+ diffusion kinetics of nickel-rich cathode materials in Li-ion batteries[J]. Journal of materials chemistry A, 2020, 8(17): 8540-8547.[4] Zhou Y, Xu G, Lin J, et al. A Multicationic-Substituted Configurational Entropy-Enabled NASICON Cathode for High-Power Sodium-Ion Batteries[J]. Nano Energy, 2024: 109812.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



