-
iestinstrument
Model Coin Cell In-situ Expansion Instrument Facilitates The R&D and Characterization Of High-capacity Silicon Anodes
1. Research Background
The current lithium-ion batteries have increasing range requirements, and it is crucial to develop anode materials with high specific capacity and high cycle stability. Silicon is the anode material with the highest theoretical specific capacity (4200 mAh/g), but its poor cycling performance due to the drastic volume change during charging and discharging greatly limits the process of large-scale commercialization.
Currently, the volume expansion of silicon anodes is mainly limited, buffered or released by nanotechnology, but the complex synthesis process, as well as the introduction of a variety of active substances have limited the practical application of this method. If the swelling/shrinking silicon materials can still be kept in close contact, the lithiation process can still be reversible, and the capacity loss due to interfacial decomposition can also be reduced.
2. Article Introduce
The team of Assistant Professor Lin Jie and Professor Peng Dongliang from Xiamen University took silicon anodes as the research object. Inspired by the anti-rust principle of stainless steel, they introduced a “bulk passivation” to stabilize the interface of silicon anodes (as shown in Figure 1), and obtained ultra-high specific capacity and cycle performance. At the same time, through non-in-situ characterization (XPS, TEM, SEM), model coin cell in-situ expansion equipment (IEST), finite element simulation, capacity differential contour map, etc., the passivation mechanisms of “single silicon”, “layer and silicon” (LiF/Li₂CO₃ coated Si), and “composite silicon” (Si/LiF/Li₂CO₃ overall composite) were systematically compared. The relevant results were published in the top international journal “Angew. Chem., Int. Ed.” with the title “Stainless Steel-Like Passivation Inspires Persistent Silicon Anodes for Lithium-Ion” and were selected as VIP articles (Very Important Paper).
![]()
Figure 1. (a) Structural damage of silicon anodes after multiple lithiation; (b) “bulk passivation mechanism” of silicon anode after multiple lithiation with the introduction of a “bulk passivation”.
3. Results and Discussion
As shown in Figure 2, the “2D composite silicon” has higher coulombic efficiency, reversible capacity and cycling performance due to the “bulk phase passivation mechanism”. The “3D composite silicon” has high specific capacity due to abundant expansion space, but the large specific surface area leads to low coulombic efficiency, while the further coated “3D composite + layer and silicon” has improved cycle performance and coulombic efficiency, and the rate performance is also extremely excellent.

Figure 2. (a) Cycling performance at 0.2C, (b) Coulombic efficiencies at 0.2C, and (c) cycling performance at 1.0C of Si anodes. (d) Rate capability of “3D composited and layered Si”.
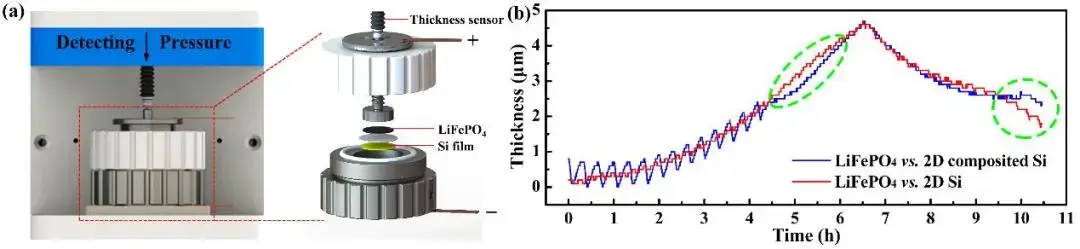
In order to compare the volume change of silicon anodes during charging and discharging before and after the modification, a model coin cell in-situ expansion instrument (IEST) was used to conduct an in-situ expansion test on a model full cell (LiFePO₄//Si), and the results are shown in Figure 3. It can be seen that the “bulk phase passivation mechanism” can effectively mitigate the volume expansion and contraction ratio of the Si anode without reducing the reversible capacity.
Figure 3. (a) In-situ swelling test setup of the full cell (IEST); (b) Swelling thickness-time curve of LiFePO₄/Si full cell
In order to further investigate the effect of “bulk passivation” on the stability of silicon anodes structure under ideal state, finite element simulation is used to compare the displacement and stress changes during expansion of “2D composite silicon” (Fig. 4a-d) and “2D monolithic silicon” (Fig. 4e-h). The finite element simulation is used to compare the displacement and stress changes of “2D composite silicon” (Fig. 4a-d) and “2D monolithic silicon” (Fig. 4e-h) during expansion. The path analysis shows that the introduction of LiF/Li₂CO₃ in the bulk phase significantly reduces the volume expansion and internal stress during lithiation, and also relieves the stress concentration phenomenon.
![]()
Figure 4. (a) Displacement variation cloud of “2D composite silicon” during expansion and its (b) path analysis, (c) stress variation cloud and its (d) path analysis; (e) Displacement variation cloud of “2D monolithic silicon” during expansion and its (f) (f) path analysis, (g) cloud of stress change and (h) path analysis.
In addition, in order to investigate the effect of “bulk passivation” on the reaction of different structures of silicon anode alloys, the capacity differential contour plots were also used to compare the redox peaks of “2D monolithic silicon”, “2D composite silicon” and “2D layer and silicon” during the long cycling. The changes of the redox peaks of “2D monolithic silicon”, “2D composite silicon” and “2D layer and silicon” during long cycling were also compared by using capacity differential contour plots, and the results are shown in Fig. 5. It can be seen that through the “bulk passivation mechanism”, the dealloying of Li₂Si and Li₃. ₇₅Si alloying reaction are both enhanced, resulting in a higher specific capacity and reversibility of the Si anode.

Figure 5. (a–c) Differential capacity plots of Si anodes from 1st to 3rd cycles. Contour maps of differential capacity vs. potential from 4th to 150th cycles upon (d–f) charge and (g–i) discharge.
In order to investigate the interfacial component changes of different structures of silicon anodes during the charging and discharging process, non-in situ XPS was used to compare the elemental changes of “2D monolithic silicon”, “2D composite silicon”, “2D layer and silicon” before, after and after the test. “The results are shown in Figure 6. The test results show that the LiF/Li₂CO₃ introduced through the bulk phase suppresses the interfacial side reactions, which improves the reaction reversibility of silicon and forms an F-rich interface stabilizing the cycling process.
![]()
Figure 6. XPS comparison charts and element contents of silicon anodes with different structures (a) before testing; (b) after discharge; (c) after charging
As shown in Figure 7, in order to more intuitively observe the structural changes of different structures of silicon anode during cycling, non-in situ SEM was used to compare the morphological changes of “2D monolithic silicon”, “2D composite silicon”, “2D layer and silicon” before testing, after discharging, and after charging. The morphological changes of “2D monolithic silicon”, “2D composite silicon”, and “2D layer and silicon” were compared by non-in-situ SEM before testing, after discharge, and after charge. The results intuitively illustrate that LiF/Li₂CO₃ introduced through the bulk phase effectively mitigates the volume change of the silicon anodes, reduces microcracks, and improves the structural stability.
![]()
Figure 7. SEM images of the surface morphology of different structures of silicon negative electrodes before (a-c) testing; after (d-f) discharge; and after (g-ì) charging
4. Summary
Inspired by the antirust mechanism of stainless steel, the performance differences of silicon anodes with different structures were systematically explored by introducing LiF/Li₂CO₃ in the bulk phase and on the surface of the silicon anode. Combined with a model coin cell in-situ swelling device (IEST), it is found that the introduction of LiF/Li₂CO₃ in the bulk phase reduces the volume change and cyclic stress of the silicon anodes, effectively suppresses the interfacial side reactions, enhances the reactivity of silicon, and creates a stabilized F-rich interface, which leads to a significant enhancement of electrochemical performance of the silicon anode. This work provides a theoretical basis and experimental evidence for the construction of stable and high-capacity anode materials through the “bulk phase passivation mechanism”.
5. References
Jie Lin, Laisen Wang, Qingshui Xie, Qing Luo, Dong-Liang Peng, C. Buddie Mullins, Adam Heller. Stainless Steel-Like Passivation Inspires Persistent Silicon Anodes for Lithium-Ion Batteries. Angewandte Chemie International Edition.
6. Related Test Equipment Recommendations
Main features:
- The instrument size is small (length *width * height: 120 * 150 * 280mm), which can be placed in the glove box;
- The model coin cell can be used to assemble various types of full coin cell;
- Good tightness can ensure long-term test stability and obtain more reliable test results;
- High-precision thickness measurement system, thickness measurement resolution 0.1um, preci.
sion ±1 um. - In-situ test of the full-cell expansion thickness curve;
- lon conductivity of the solid electrolyte can be measured;
- The software can automatically combine the thickness change of the model coin cell with thecharging and discharge data (compatible with partial charging and discharge tester), and outputthe report involving all testing data.
Main features:
- In-situ characterization of the expansion thickness change of the cell;
- Four-channel synchronized testing of multiple battery cells;
- Adapt to the in-situ expansion test of a variety of cell structures: model buckled battery, laminated battery, pouch cell, prismatic cell, etc.;
- High-precision thickness measurement system, thickness measurement resolution 0.1µm, accuracy ±1µm;
- Can test the solid state electrolyte ionic conductivity;
- The software automatically merges the model battery thickness change data and charging and discharging data (compatible with some charging and discharging equipments), and outputs the test data report.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.