-
iestinstrument
Characterization and Quantification of Multi-field Coupling in Lithium-ion Batteries Under Mechanical Constraints

Research Topics:Characterization and Quantification of Multi-field Coupling in Lithium-ion Batteries Under Mechanical Constraints
First Author: Xue Cai
Corresponding Authors: Caiping Zhang, Weihan Li
Affiliated Institutions: Beijing Jiaotong University, RWTH Aachen University
Equipment Used: IEST SWE2110 (1T Room Temperature Expansion Testing System)
1. Research Background
With the widespread adoption of electric vehicles and electrochemical energy storage, lithium-ion batteries have garnered significant attention due to their high energy density, cost-effectiveness, and long lifespan. However, during electrochemical reactions, lithium-ion batteries generate expansion forces and heat, particularly under mechanical constraints such as assembly and stacking. This leads to electrochemical-thermal-mechanical multi-field coupling behavior.
Characterizing and quantifying multi-field coupling behavior requires interdisciplinary efforts. However, due to measurement limitations and the complexity of the coupling, understanding the intricate multiphysics behavior presents two challenges. First, although existing testing platforms provide experimental means to study battery mechanical responses and multiphysics coupling behavior, each platform has its advantages and disadvantages. Second, the highly nonlinear interactions in the intricate multi-field coupling behavior have not been thoroughly decoupled and analyzed. Therefore, this paper leverages the strengths of different mechanical constraint platforms to develop an in-situ quantitative analysis method to elucidate the mechanisms of multi-field coupling, quantifying the correlations and coupling strengths between different physical fields. These research findings will provide crucial insights for optimizing structural design and improving battery performance.
2.Work Overview
Recently, a team from Beijing Jiaotong University, in collaboration with RWTH Aachen University, designed a series of mechanical constraint experiments, including free expansion (Figure 1(a)), constant displacement (Figure 1(b)), and IEST SWE2110 constant pressure mode (Figure 1(c)). An in-situ analysis framework was introduced to elucidate the complex interaction mechanisms and coupling degrees between multiple physical fields. The proposed analysis framework integrates equivalent model parameterization, in-situ mechanical analysis, and quantitative assessment of coupling behavior.
The results indicate that at low temperatures, the significant impact of pressure on impedance primarily originates from the diffusion-controlled steps, and applying external pressure (e.g., 180 to 240 kPa at 10°C) can improve the battery’s kinetic performance. The diversity of electrochemical reaction control steps demonstrates the varying effects of pressure on battery performance at different temperatures. The thermal expansion rate shows that during charging, the change in expansion force per unit temperature increase is less than 1.60%. By introducing composite evaluation metrics, we quantified the coupling correlations and strengths between characteristic parameters and physical fields, revealing the highest coupling degree between the electrochemical and thermal fields. These findings highlight the potential of the analytical method in uncovering multiphysics interaction mechanisms, aiming to enhance battery performance and optimize structural design.
3. Content Description
Lithium-ion batteries involve various disciplines and nonlinear coupling behaviors, making the analysis of multiphysics problems evidently intricate. In this study, we propose an in-situ quantitative analysis framework as illustrated in Figure 1, aimed at comprehensively addressing these complex nonlinear coupling analysis issues. To address the battery temperature, which cannot be measured under mechanical constraints, we integrate three mechanical platforms and carefully design a comprehensive experimental matrix to provide data support for multiphysics model parameterization and mechanical characteristic curve analysis.
Firstly, to quantitatively evaluate the intricate interactions between different fields, we visualized and parameterized the coupling behavior using equivalent circuit, mechanical, and thermal models (Figure 1(d)), providing a crucial basis for intuitively quantifying the multiphysics coupling between characteristic parameters and physical fields. While the influence of thermal effects on mechanical behavior was explored using a one-way coupling approach, the interactions between other physical fields required a two-way coupling strategy. Subsequently, to further investigate the effects of temperature and pressure on mechanical behavior, we developed an in-situ mechanical characterization method using differential voltage and expansion analysis (Figure 1(e)). With this approach, we could correlate expansion peaks with specific phase transitions, thereby exploring the electrochemical characteristics of mechanical behavior variations.
Additionally, we introduced a comprehensive metric combining Maximum Information Coefficient (MIC) and Maximum Rate of Change (MRC) to quantitatively assess the coupling correlation and strength between characteristic parameters and physical fields (Figure 1(f)). This study emphasizes the guidance provided by this framework in experimental design for addressing multi-field coupling issues, aiming to eliminate interactions and create models specifically tailored to address the challenges of multi-fieldcoupling.
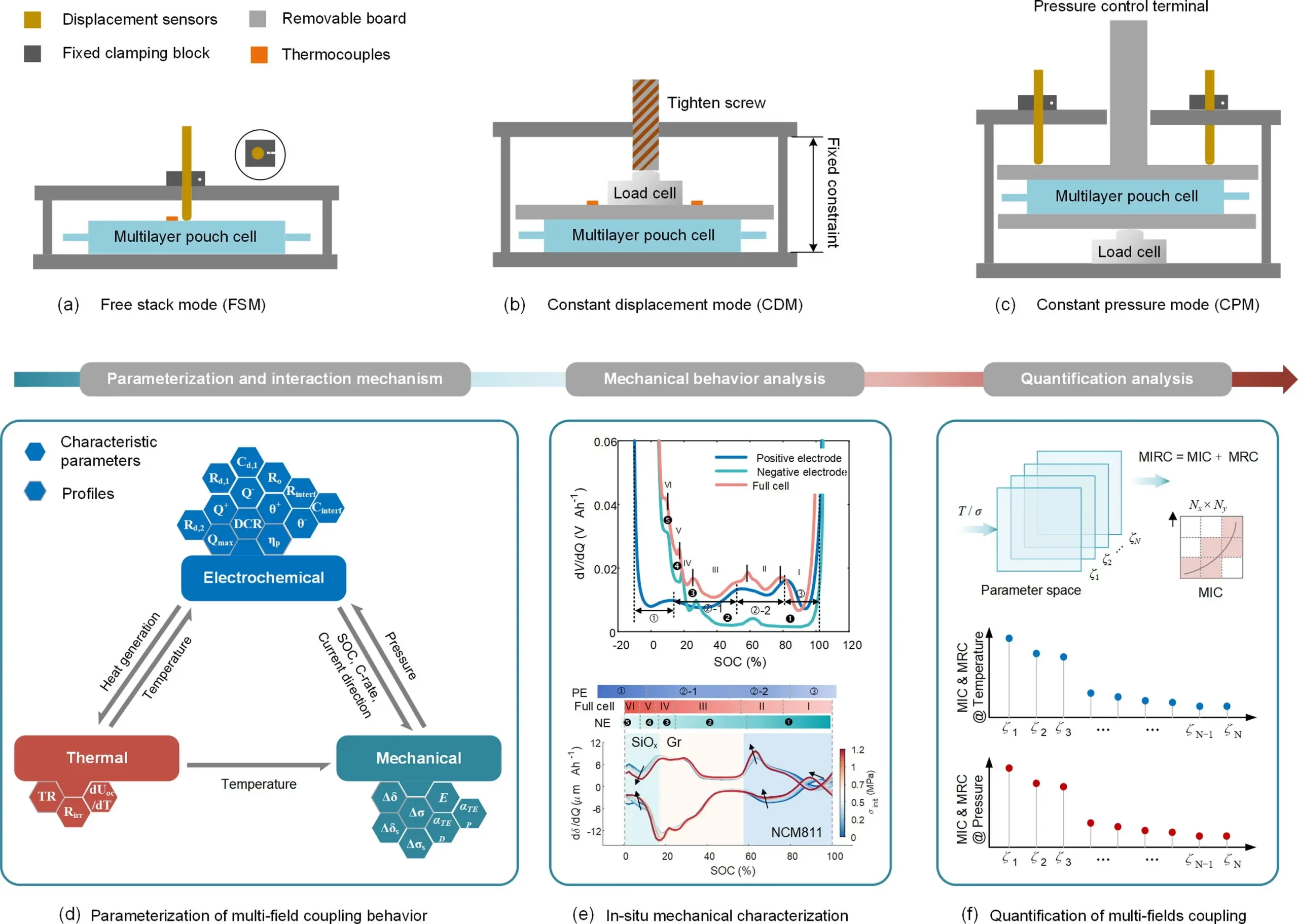
Figure 1. Schematic overview of test platforms and in-situ quantitative analytical framework.
To further elucidate the internal electrochemical mechanisms underlying the external performance variations of the battery, Figure 2 illustrates the variations of thermodynamic and kinetic parameters of the battery with external pressure at different temperatures. The thermal expansion of active material particles promotes a tight connection between particles, binder, and conductive materials. Due to the absence of voids, this results in the electron impedance remaining unchanged as pressure increases. Conversely, at 10°C, the loose connections of these particles lead to a decrease in electron impedance with increasing pressure due to the elimination of voids. In this scenario, the change in electron impedance caused by stress is significantly greater than that of lithium-ion impedance, indicating enhanced contact status between components in the pressurized cells at 25°C and 10°C.
In Figure 2 (d and e), it can be observed that at all temperatures, with increasing pressure, the interface impedance parameters, Rinterf decrease while Cinterf increases. This indicates that the surface area of active material particles increases with applied pressure, but at pressures exceeding 100 kPa at 40°C, and below 50 kPa at 20% SOC, the surface area decreases due to mechanical damage (such as particle embedding into the current collector or secondary particle fusion) leading to a reduction in active material surface area [54]. Therefore, considering the entire SOC range, the optimal pressure for maximizing active surface area (i.e., minimizing interface impedance) is concentrated within the range of 50 to 100 kPa at 40°C, 100 kPa at 25°C, and 240 kPa at 10°C, providing strong theoretical support for buffering layer-supported long-term operation of the battery. In Figure 2 (f-h), the low-frequency impedance is composed of ion diffusion within the electrolyte and active material particles. Due to the reduction in porosity, the diffusion impedance, Rd,1, increases with stress at 25 and 40°C, while it decreases at 10°C, attributed to the correlation between the diffusion coefficient at 10°C and the electrolyte viscosity or even solidification induced by stress, indicating a significant impact of pressure on battery impedance performance under low-temperature conditions.
Liquid-phase diffusion is the key controlling step for low-temperature performance [56], contributing to the improvement of kinetics by applying external pressure. According to the electrochemical principles of the battery, variations in electrochemical reaction control steps can effectively explain different effects at different temperatures. The mechanisms of pressure-induced kinetic evolution and changes in electrode capacity in thermodynamics reveal that the essence of the increase in battery 1.5C capacity and decrease in polarization voltage at 10°C by applying pressure originate from pressure-induced diffusion-controlled steps.
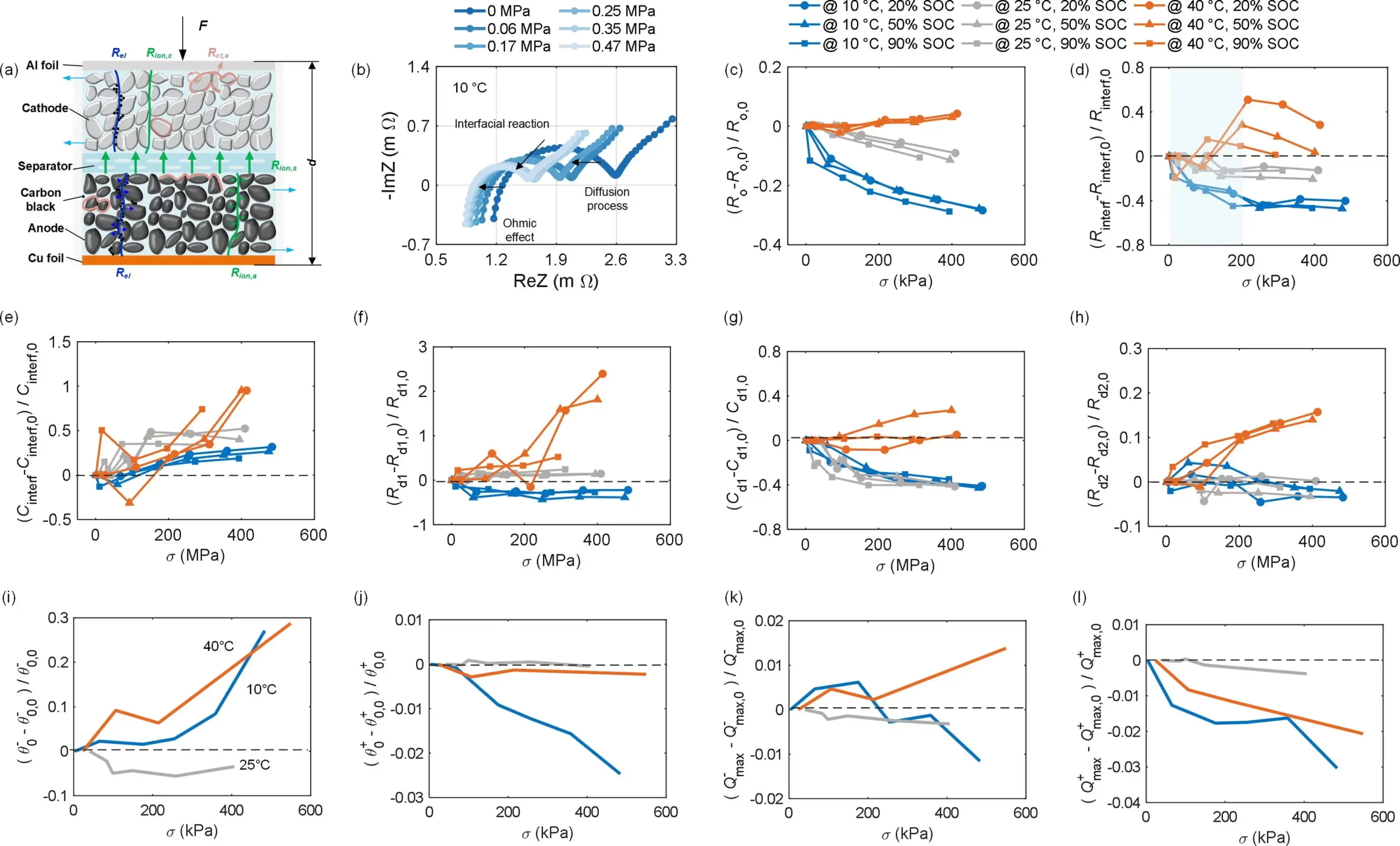
Figure 2. (a) Nyquist plot of EIS. (b) Schematic diagram of the effect of pressure on electrochemical properties. The resistance from the initial value (R0) under low stack stress is calculated from comparison. (c) Ohmic resistance. (d) Interfacial resistance. (e) Interfacial capacitance. (f) Electrolyte diffusion resistance and (g) capacitance. (h) Other diffusion resistance. The stress-induced thermodynamic parameters. Thermodynamic parameters include the initial lithiation state of (i) anode and (j) cathode. (k) Anode and (l) cathode capacity.
In Figure 3 (b), the battery thermal expansion force TEPc is linearly correlated with temperature, and the thermal expansion coefficient αTEP increases with increasing SOC, ranging from 0.70 to 0.87 kPa/°C. To analyze the magnitude of thermal expansion stress, we propose a new parameter λTEP to represent the ratio of αTEP to the lithiation-induced expansion force Δσs at 25°C. The variation of λTEP is less than 1.60%, indicating that the pressure change induced by TR at 10°C is less than 16%. Figure 3 (b) demonstrates that the battery’s thermal expansion increases or decreases linearly with temperature when the gap between electrode layers is filled above 25°C. However, an opposite trend to thermal expansion displacement TEDc and TEPc is observed at 20% SOC, indicating that different experimental results may occur due to the different contact states between electrodes under mechanical constraints. Additionally, to further analyze the extent of thermal expansion during actual battery operation and its correlation with SOC, we designed 1 C charging and 1.5 C discharging expansion tests with or without temperature rise TR. As shown in Figures 3 (c and d), at the end of charging, a TR of 2.28% leads to a 2.16% increase in expansion, while at the end of discharging, a TR of 1.21% results in a 4.34% decrease in expansion. These conclusions provide a basis for understanding thermal-mechanical coupling behavior and elucidate the necessity of experimental design under mechanical constraint conditions.
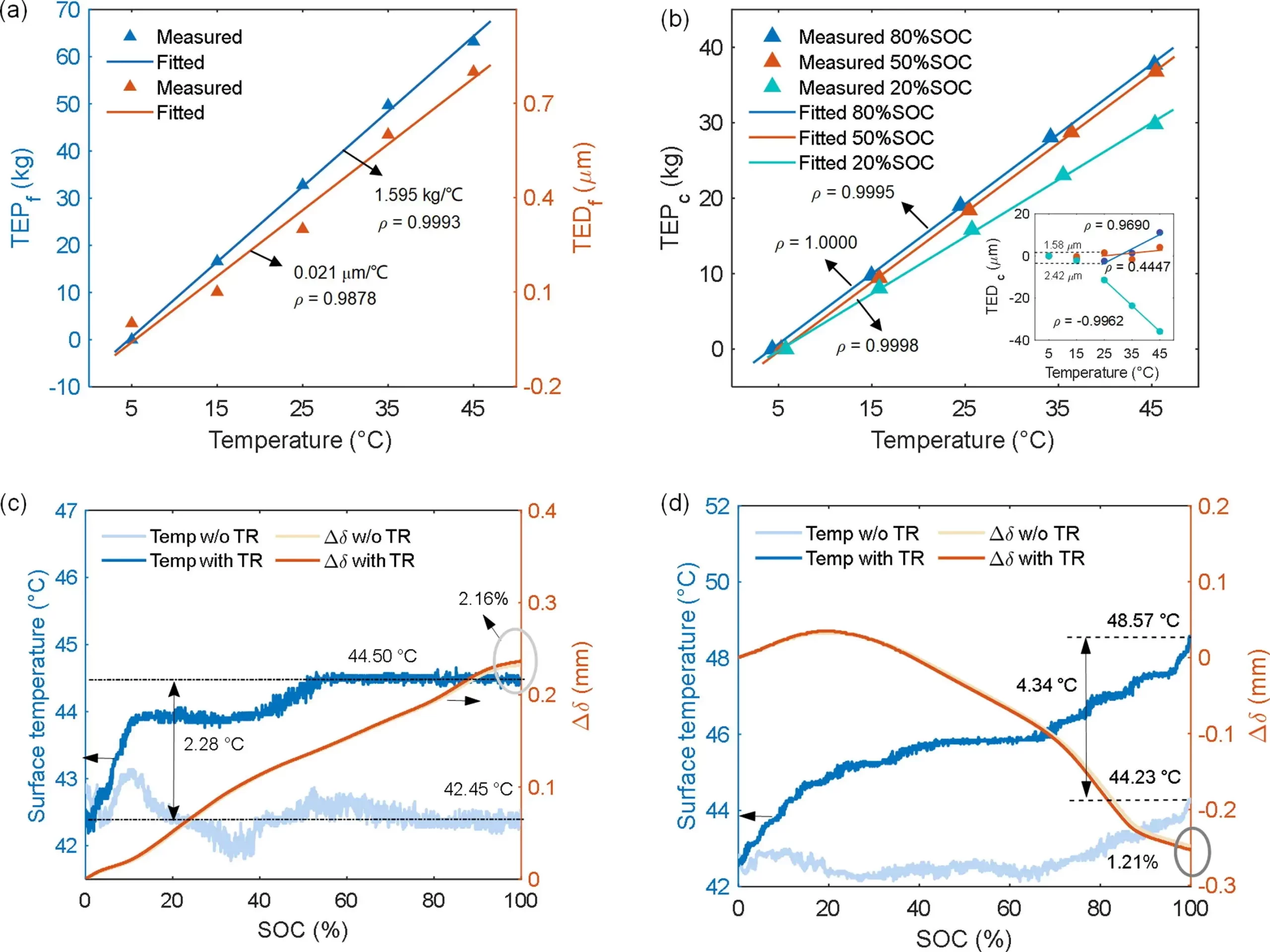
Figure 3. Thermal expansion characteristic analysis. (a) Thermal expansion pressure of the combined fixture and sensors. (b) Cell thermal swelling pressure and displacement at 20%, 50%, and 80% SOC. The change of temperature and swelling thickness with and w/o TR at (c) 1 C charge and (d) 1.5 C discharge.
In the context of multi-field coupling problems, due to the complexity and diversity of coupling relationships, it is necessary to further quantify the correlation and dependency between two fields. Figures 4 (a) to (d) display the normalized MIC and MRC between characteristic parameters and physical fields such as temperature (T) and pressure (σ). In Figure 11 (a), except for Q+max, the temperature correlation of all electrochemical parameters exceeds 0.6 Temp@MIC, while the correlation of mechanical parameters with temperature is the weakest. An interesting phenomenon in Figure 11 (c) is that all mechanical parameters are highly correlated with pressure, while the Pres@MIC between pressure and electrochemical parameters is less than 0.6. Although correlation assessments based on MIC indicate some connection between these two domains, the degree of their interaction remains undetermined, which is crucial for assessing battery performance reliability and estimating algorithm robustness.
For the analysis of coupling strength, as shown in Figure 11 (b), the temperature-induced characteristic parameters Temp@MRC suggest that improving the temperature characteristics of the electrolyte and increasing the active area of the solid-liquid reaction interface are effective ways to enhance battery capacity and power performance. The pressure-induced mechanical parameters Pres@MRC indicate that Δδ has lower sensitivity to temperature and pressure, while Δσ has higher sensitivity to pressure and moderate sensitivity to temperature. In practical applications, pressure signals are easier to obtain than strain sensors with lower accuracy. Therefore, it is more reasonable to establish a high-precision mechanical model with pressure as input and strain as output, which helps achieve online diagnosis of early battery failures. During battery operation, the thermal-electrical coupling is more pronounced than the other two field couplings. This conclusion aids in designing decoupling experiments and developing multi-field coupling modeling methods to ensure the reliability of BMS functionality during the actual operation of electric vehicles.
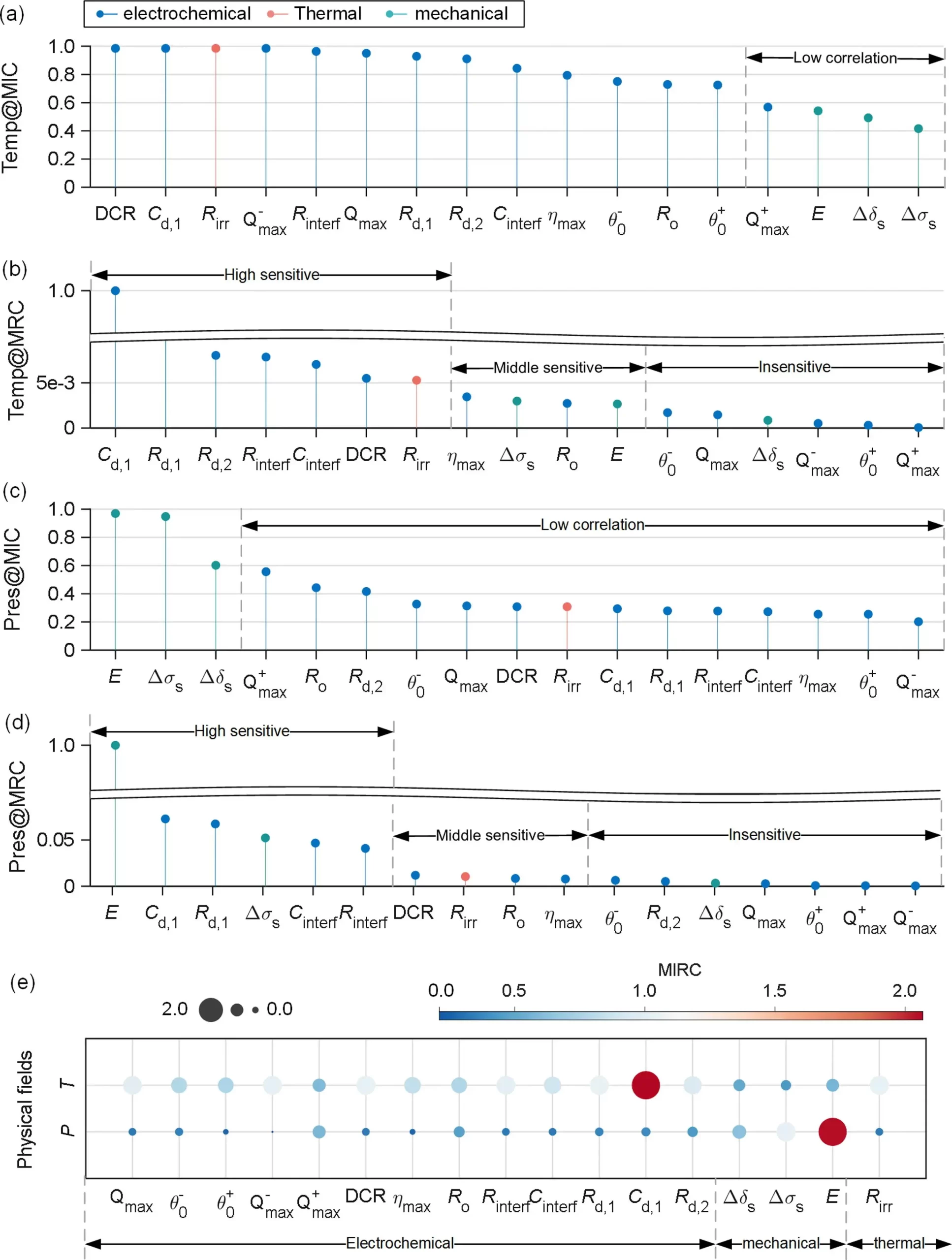
Figure 4. (a) Normalized MIC and (b) MRC for temperature-induced parameters. (c, d) Pressure-induced parameters. (e) Bubble plot for quantification of multi-field coupling by composite MIRC. The parameters are categorized into three types: electrochemical, mechanical, and thermal parameters.
4. Summary and Prospects
This paper proposes an analytical framework combining mechanical constraint experiments with an in-situ quantitative framework. By directly measuring multi-physics signals under three mechanical constraint conditions, a comprehensive characterization dataset is created to reveal multi-field coupling mechanisms, separate the influences of SOC, temperature, and pressure on mechanical behavior, and quantify the coupling degree between multiple physical fields. The results indicate a close correlation between coupling behavior and battery electrochemical characteristics. Due to changes in key control steps, temperature leads to varying degrees of pressure-induced impedance changes and different heating and performance improvement effects. The variation in interface impedance closely associated with active surface area further demonstrates the relationship between optimal pressure ranges and temperature, such as 180 to 240 kPa at 10°C, 100 to 150 kPa at 25°C, and 50 to 100 kPa at 40°C. In the thermal-induced mechanical behavior at 20% SOC, different degrees of thermal expansion are induced by mechanical constraints due to the increase in elastic modulus with temperature rise. During the charging process, a 1.60% expansion force is generated per unit temperature increase. Quantitative results from MIC and MRC indicate a high correlation between electrochemical parameters and temperature, while mechanical parameters exhibit strong correlation with pressure. The sensitivity of all electrochemical parameters to temperature is much higher than that to pressure, indicating the highest coupling degree between electrochemical and thermal fields.
This work highlights the potential of analysis procedures in visualizing and quantifying multi-field coupling, providing deeper insights into coupled interaction mechanisms, and offering solutions for multi-field simulation. These advancements will inevitably guide the improvement of battery performance and optimization of structural design.
X.Cai, C. Zhang, Z. Chen, L. Zhang, D. Uwe Sauer, W. Li, Characterization and quantification of multi-field coupling in lithium-ion batteries under mechanical constraints, Journal of Energy Chemistry (2024), doi: https://doi.org/10.1016/j.jechem.2024.03.048
 |
Xue Cai, a doctoral student at the School of Electrical Engineering, Beijing Jiaotong University, is jointly enrolled as a doctoral student at RWTH Aachen University in Germany. Her research focuses on optimizing the performance and safety management techniques of dynamic/energy storage batteries under mechanical pressure. |
 |
Caiping Zhang, a professor at the School of Electrical Engineering, Beijing Jiaotong University, and the Deputy Director of the Ministry of Education Engineering Research Center for Intelligent Traffic Green Low-Carbon Technology. She has long been engaged in research on optimization control and safety management techniques for dynamic/energy storage batteries, and has led multiple projects funded by the National Natural Science Foundation of China and the National Key Research and Development Program. She has published over 70 SCI journal papers and has been awarded the Second Prize of National Science and Technology Progress Award, the First Prize of Science and Technology Invention Award of the Ministry of Education, and the National Science Foundation for Excellent Young Scholars. |
 |
Weihan Li is the leader of the “Battery Artificial Intelligence” young research team at RWTH Aachen University. He obtained his doctoral degree in Electrical Engineering and Information Technology in 2021 and his master’s degree in automotive engineering in 2017 from RWTH Aachen University. Weihan Li has conducted research at Imperial College London, the University of Oxford, the Massachusetts Institute of Technology, Volkswagen Group, and Porsche Group in Germany. He has received multiple awards, including the BattFutur Starting Grant from the German Federal Ministry of Education and Research, the German Research Prize from the Koerber Foundation, the Reichart Prize from the Erfurt Academy of Sciences, the Innovation Prize from the vgbe Foundation in Germany, the European Battery Young Researcher Award, and the Innovation Award from RWTH Aachen University |
Updated on Jan. 14, 2025
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


