-
iestinstrument
Entropy Surface Engineering Promotes The Stability Of Multi-electron Reactions Of Anions And Cations In Li-rich Layered Cathodes
 Literature Appreciation: Entropy Surface Engineering Promotes The Stability Of Multi-electron Reactions Of Anions And Cations In Li-rich Layered Cathodes
Literature Appreciation: Entropy Surface Engineering Promotes The Stability Of Multi-electron Reactions Of Anions And Cations In Li-rich Layered Cathodes
First Author: Jiayu Zhao
Corresponding Authors: Yuefeng Su, Jinyang Dong, Lai Chen
Affiliations: Beijing Institute of Technology, Chongqing Innovation Center of Beijing Institute of Technology, Initial Energy Science & Technology(IEST) Co.,Ltd,
Equipment Used: IEST In-Situ Battery Gassing Volume Analyzer (GVM2200)
1. Research Background
Cobalt-free lithium-rich manganese-based layered oxides (LMROs) are considered efficient cathode materials for lithium storage due to their high specific capacity and cost-effectiveness. However, structural degradation leads to poor cycling and rate performance, hindering their widespread application. Essentially, rapid structural degradation stems from irreversible anionic redox reactions, triggering oxygen release, transition metal migration, and electrolyte decomposition. This study proposes a strategy to construct an epitaxial entropy-assisted layer, where the restructured cathode material surface comprises various heterogeneous element dopants and composite microstructures, ensuring structural stability during cycling. Multi-site heteroatom doping effectively regulates the chemical environment and electronic structure of lattice oxygen, inhibiting oxygen release and transition metal migration, thereby reducing structural degradation. The composite microstructure of oxygen vacancies and spinel phases promotes surface lithium transport while reducing surface lattice oxygen release and electrolyte decomposition. This entropy-assisted surface engineering provides an effective pathway for stabilizing high-energy cathode materials.
2. Article Summary
Recently, Professor Yuefeng Su and Researcher Lai Chen from Beijing Institute of Technology published a research article titled “Regulating anionic redox reversibility in Li-rich layered cathodes via diffusion-induced entropy-assisted surface engineering” in the internationally renowned journal Energy Storage Materials. This work proposes diffusion-induced entropy-assisted (DIEA) surface engineering to enhance the cycling stability of cathode materials, promoting the practical application of li-rich layered cathodes in high-energy-density, long-life batteries.
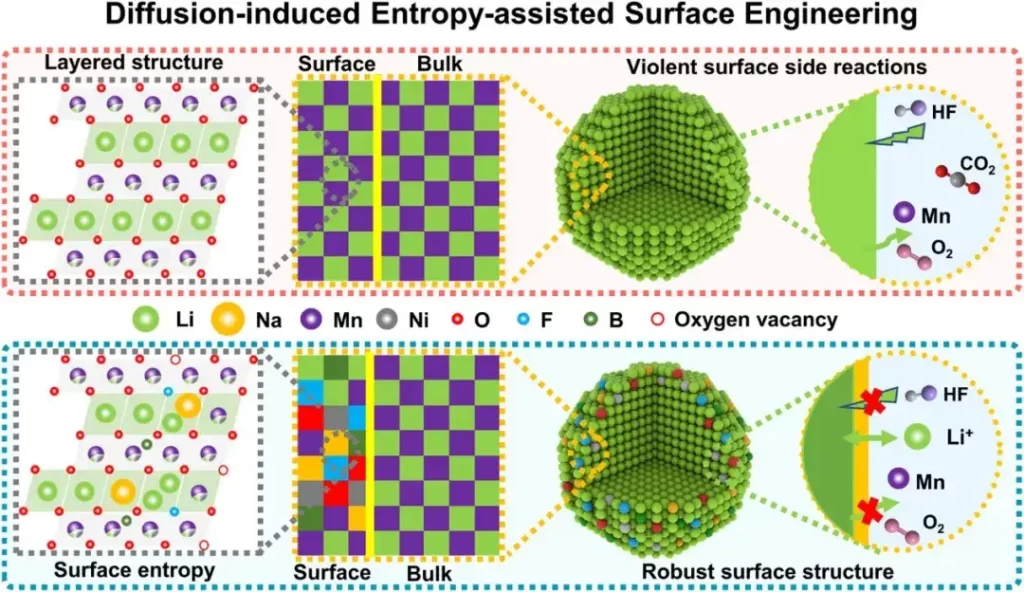
Figure 1. Schematic illustration of the DIEA strategy modification mechanism
3. Key Points
3.1 Key Point 1: Diffusion-Induced Entropy-Assisted (DIEA) Surface Engineering
Li-rich layered cathodes materials exhibit complex cationic and anionic redox reactions. During cycling, irreversible structural changes (oxygen framework disruption, transition metal migration) lead to performance degradation. In this study, DIEA engineering is adopted to construct a composite surface phase on lithium-rich manganese-based cathodes. At the atomic scale, heterogeneous elements (Na, F, B) respectively substitute Li, O, and tetrahedral sites, enhancing surface configurational entropy by increasing anionic and cationic composition to stabilize the structure. In terms of crystal configuration, new diversified configurations such as “Li-O-Na,” “Li-F-Li,” and “Li-F-Na” regulate the redox barriers of lattice oxygen, mitigating irreversible lattice oxygen release. At the surface phase composition scale, the introduced spinel phase and oxygen vacancies accelerate interface Li+ transport and reduce lattice oxygen release. Overall, the high-entropy surface constructed by DIEA engineering increases structural diversity at multiple scales, regulates cationic and anionic redox reversibility, and stabilizes the crystal structure.
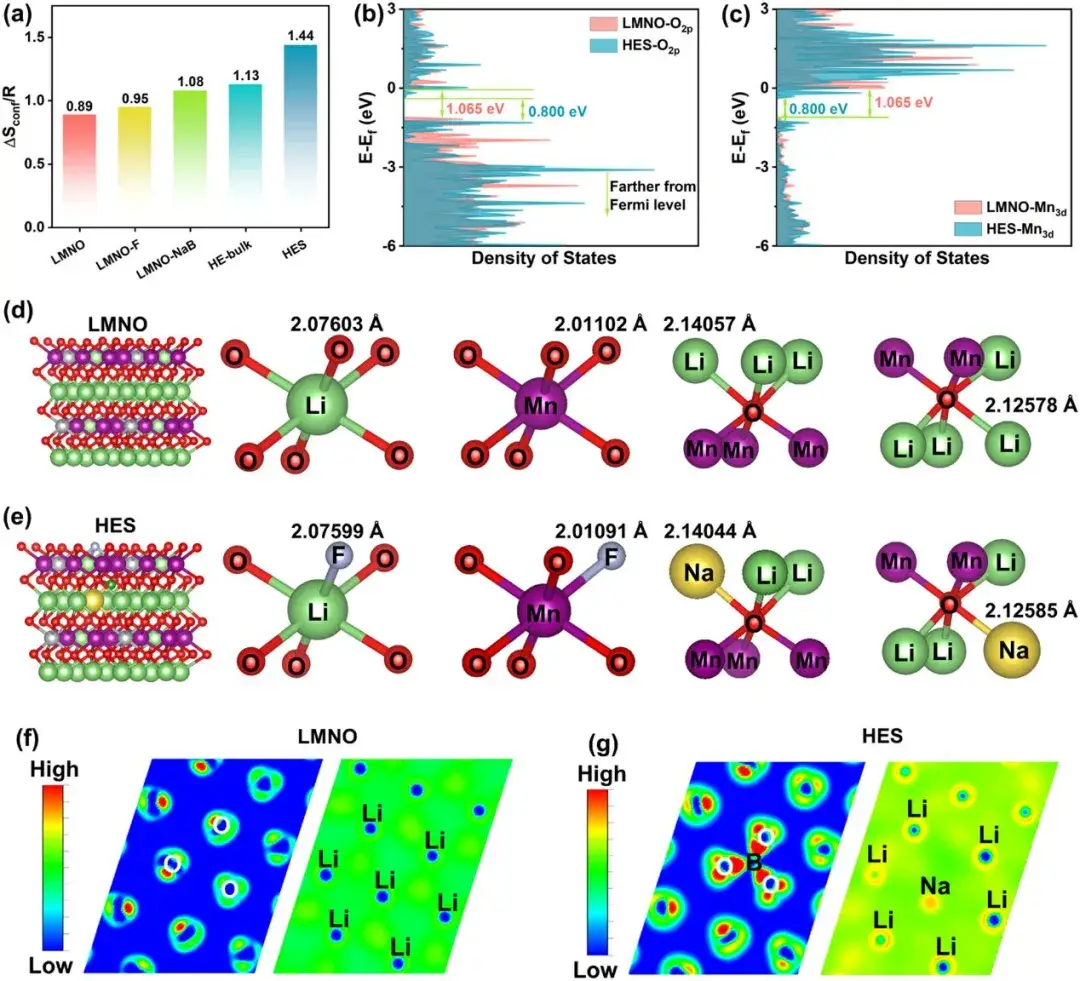
Figure 2. (a) Configurational entropy of different element compositions; (b) Density of states of O2p and (c) Mn3d orbitals; (d) Octahedral configurations and bond lengths in LMNO and (e) HES samples; (f) 2D charge density distribution in LMNO and (g) HES samples
3.2 Key Point 2: Regulation of Cationic and Anionic Evolution
To investigate the regulation of lattice oxygen release and transition metal migration by DIEA engineering, in-situ XRD, in-situ Raman, and in-situ DEMS tests were conducted. In-situ XRD tests show that the cell parameters of the material change slowly at high cutoff voltages, and the crystal structure stabilizes with reduced strain. In-situ Raman tests analyze the transition metal migration and structural degradation in the samples. Modified samples show minor changes in characteristic peaks at 500 cm-1 and 618 cm-1, while new peaks at 650 cm-1 appear in the original samples, indicating new spinel phases formed due to transition metal migration. In-situ DEMS tests demonstrate that the high-entropy surface significantly suppresses gas release at high voltages, indicating a more stable lattice oxygen framework. Further DFT calculations provide theoretical support for lattice oxygen release and transition metal migration, showing that high-entropy surface materials exhibit higher oxygen vacancy formation energy and Mn migration barriers in highly delithiated states, consistent with test results, indicating a more stable crystal structure at high pressures.
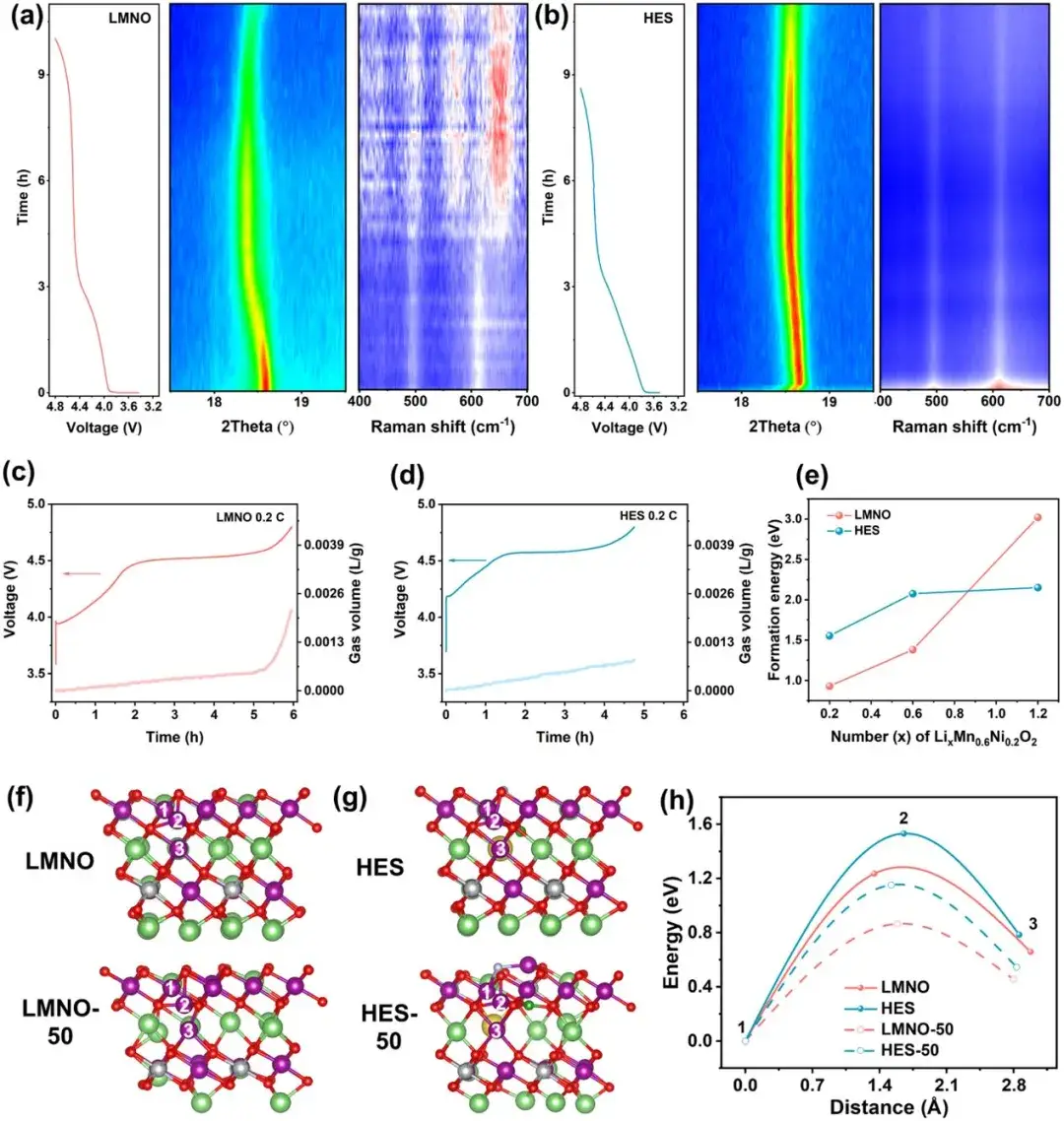
Figure 3. In-situ XRD and in-situ Raman test results of (a) LMNO and (b) HES samples; In-situ gas release curves of (c) LMNO and (d) HES; Oxygen vacancy formation energy of different samples (e); Transition metal migration paths in different delithiated states of (f) LMNO and (g) HES; Mn migration energy in different samples (h)
3.3 Key Point 3: Enhancing Structural Stability and Mitigating Interface Side Reactions
Tests on the cathode materials after long cycles prove the effectiveness of DIEA engineering. Tests such as sXAS, Raman, TEM, and XRD validate the structural and compositional changes during electrochemical cycling. sXAS surface detection of different samples after cycling shows that high-entropy surface materials exhibit higher lattice oxygen redox reversibility, higher transition metal oxidation states, and the pre-constructed high-entropy surface remains stable after cycling. ICP-OES tests of the electrolyte and lithium after cycling indicate that the high-entropy surface mitigates the dissolution of transition metals from the cathode material and deposition on the anode surface, reducing interface side reactions and cross-talk effects. Raman, TEM, and XRD analyses of the crystal structure on the surface and bulk phases show that the high-entropy surface mitigates irreversible phase transitions, reduces stress accumulation in the particles, and enhances crystal structure stability.
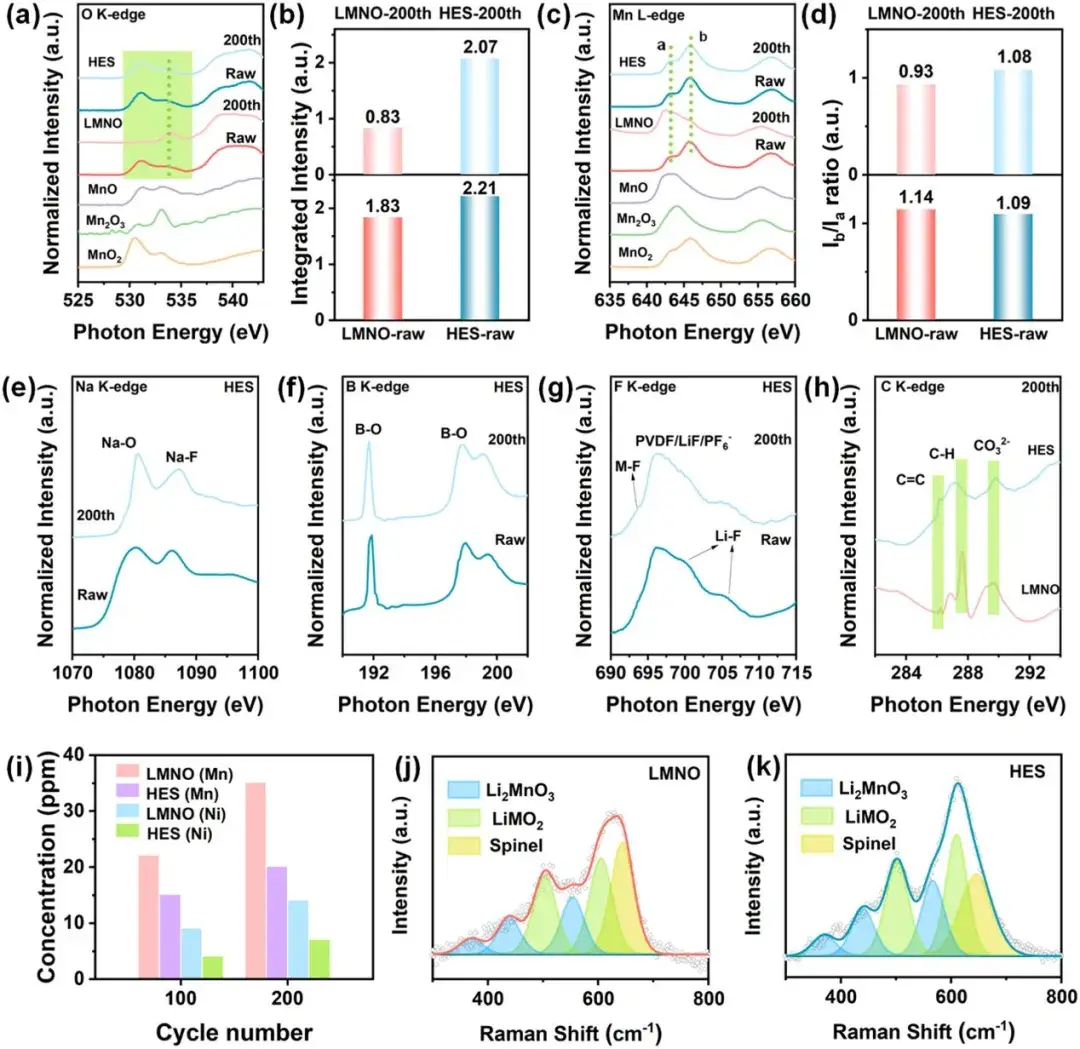
Figure 4. TEY mode (a) O K-edge and (b) corresponding shaded area integral intensity; TEY mode (c) Mn L-edge and (d) corresponding peak intensity ratio; Pre- and post-cycling absorption spectra of (e) Na K-edge, (f) B K-edge, (g) F K-edge, (h) C K-edge; Transition metal concentration in post-cycling electrolyte (i); Raman spectra of LMNO (j) and HES (k) after cycling
3.4 Key Point 4: Looking ahead
In this work, we constructed a high-entropy surface phase on li-rich layered cathodes through DIEA engineering. This surface phase combines spinel/oxygen vacancy phases and multi-site heterogeneous element doping regions, increasing local structural diversity, thus enhancing the energy barriers for lattice oxygen release and transition metal migration, thereby inhibiting structural degradation. This method elucidates the mechanism of this modification strategy and verifies the effectiveness of high-entropy strategies in optimizing the performance of li-rich layered cathodes materials. This method can be extended to other cathode materials, promoting the further development of high-energy-density, long-life lithium-ion batteries.
Link to the Article: Regulating anionic redox reversibility in Li-rich layered cathodes via diffusion-induced entropy-assisted surface engineering
4. Corresponding Authors’ Profiles
Lai Chen
Researcher and PhD supervisor at the School of Materials, Beijing Institute of Technology. Selected for the 4th China Association for Science and Technology Young Talents Lift Project and the Beijing Science and Technology New Star Program. Currently focuses on lithium-ion secondary batteries and other electrochemical energy storage materials and devices, with research directions including li-rich layered cathodes materials, high-nickel cathode materials, and high-energy-density lithium-ion secondary batteries. As a principal investigator, he has led over 10 projects including the National Key Research and Development Program, National Natural Science Foundation, and projects from Yibin Science and Technology Bureau. Since 2013, he has published over 60 SCI papers in international journals such as Advanced Materials, Advanced Energy Materials, and Nano Energy, and has received 65 national invention and utility model patents, with 32 granted. He has published three monographs.
Yuefeng Su
Professor and PhD supervisor at the School of Materials, Beijing Institute of Technology, and responsibility professor at the Academician Center for New Materials, Chongqing Innovation Center of Beijing Institute of Technology. In 2013, he was selected for the Ministry of Education’s “New Century Excellent Talents Support Program” in the field of new materials. His research focuses on green secondary batteries and advanced energy materials. As a project leader, he has led two National Natural Science Foundation projects, one National Key Research Task, and one international cooperation project. He has participated in several projects including the “973” Program, major projects on “New Energy Vehicles,” and other National Natural Science Foundation projects. As a corresponding author, he has published over 90 SCI papers in journals such as Advanced Materials, Nano Energy, Energy Storage Materials, Nano Letters, and Journal of Materials Chemistry A, and has applied for nearly 60 national invention patents, with over 30 granted.
Jinyang Dong
Postdoctoral researcher at the School of Materials, Beijing Institute of Technology, working with Academician Feng Wu. His research focuses on the modification of lithium-ion battery cathode materials and accelerated aging failure analysis of energy storage batteries. As a primary researcher, he has participated in projects including the National Key Research and Development Program and projects from Yibin Science and Technology Bureau. He has published 14 SCI papers in international journals such as Advanced
6. Related Test Equipment Recommendation:
IEST In-Situ Battery Gassing Volume Analyzer (GVM2200) , test temperature range of 20℃ ~85℃, support dual-channel (2 cells) synchronous test, 1L resolution, good long-term stability, can synchronously monitor the gas production volume change of cells under circulation, storage, overcharge and discharge and other conditions, to help the research and development of materials and cells.

Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


