-
iestinstrument
Dynamic Hydrogen Bonding Binder with Self-Healing Capability Enables Development of High-Performance Silicon Anode Lithium-Ion Batteries
1. Article Abstract
Structural instability and stress accumulation-induced cycling degradation of silicon anodes have hindered their practical application in next-generation high-energy-density lithium-ion batteries (LIBs). Recently, M.S. Jiahao Chen and Prof. Hui Yang from Nanjing University of Technology (NUIST) developed a crosslinked polymer as a self-healing silicon anode binder by in-situ polymerization of tannic acid (TA) and poly(acrylic acid) (PAA) binder (denoted as TA-c-PAA). The branched TA acts as a physical cross-linking agent to combine with the PAA backbone through abundant dynamic hydrogen bonding, which gives the cross-linked TA-c-PAA binder unique self-healing properties and strong adhesion to the silicon anode. Thanks to the mechanical robustness and strong adhesion, the Si@TA-c-PAA electrode has a high reversible specific capacity (3250 mAh/g at 0.05C multiplication rate (1C=4000 mA/g)), excellent multiplication capability (1599 mAh/g at 2C multiplication rate), and impressive cycling stability (after 450 cycles, the 0.25C specific capacity of 1742mAh/g at 0.25C magnification). After non-in-situ morphological characterization, in-situ swelling analysis and finite element simulation, it was found that the TA-c-PAA binder can enable the silicon anode to disperse stress and prevent particle crushing during lithium embedding and delithiation, and that hydrogen bonding between the interpenetrating networks can adapt to the stress intensity. This work paves a new way for the design of efficient and low-cost binders for silicon anodes of next-generation lithium-ion batteries.
The related results were published in the internationally renowned journal “J Colloid Interf. Sci.” under the title “Dynamic hydrogen bond cross-linking binder with self-healing chemistry enables high-performance silicon anode in lithium-ion batteries“.
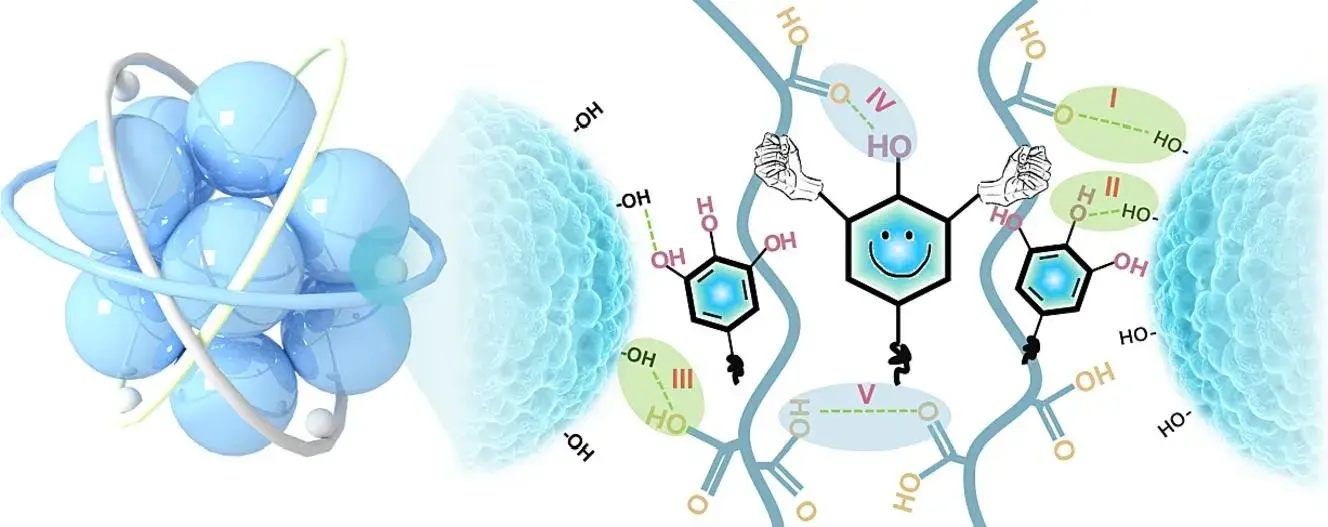
Figure 1. Three-dimensional flexible self-healing adhesive network of polyacrylic acid and tannic acid crosslinked by intermolecular hydrogen bonding
2. Original Paper Appreciation
The schematic of the synthesis process and molecular structure of TA-c-PAA polymeric binder are shown in Figure 2. The three-dimensional cross-linked binder was synthesized by free radical reaction using acrylic acid as the polymerization monomer and potassium persulfate as the initiator. Among them, PAA provides the main mechanical support for the main skeleton of the polymer network and has a strong adhesion ability to Si. The authors used tannic acid as the physical cross-linking agent and constructed TA-c-PAA to form an interpenetrating cross-linking network through dynamic hydrogen bonding, and it has self-healing ability and excellent mechanical properties. The preparation process of this method is feasible and easy to scale up the production.
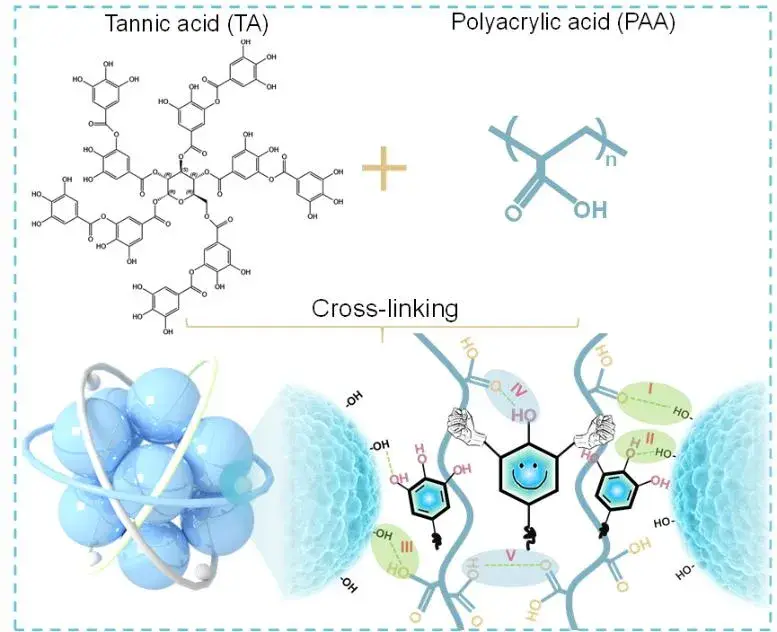
Figure 2. Synthesis process of crosslinked TA-c-PAA binder and mechanistic study
Figure 3(a) shows the FTIR test results of three substances: PAA, TA and TA-c-PAA. The position of C=O moves from 1720 cm-1 of PAA to 1740 cm-1 of TA-c-PAA. It shows that there is (P, C=O…H-O-Ph) bond between TA and PAA. The 1H NMR of TA and TA-c-PAA binders were further analyzed to study the distribution and state of protons within the polymer binder molecule (as shown in Figure 2(b)). Compared with TA and PAA, the proton peak shift of TA-c-PAA is larger, indicating that there are more electronegative elements or chemical groups near the proton, and the reduction in electron cloud density leads to a larger shift, further explaining The formation of intermolecular hydrogen bonds.
The glass transition temperatures (Tg) of PAA and TA-c-PAA were further studied using DSC, and the results are shown in Figure 3(c). The results show that the Tg value of TA-c-PAA is much lower than that of PAA, indicating that weak hydrogen bonds are formed between PAA and TA, which weakens the interaction within the molecular chain and improves the toughness of the polymer binder. To examine the electrochemical stability of the above-mentioned polymer binder, the author further tested cyclic voltammetry (CV) in the range of 0.01 ~ 1.0 V using buckle current, and the results are shown in Figure 3(d). Within the tested voltage range, no obvious redox peak appears for PAA and TA-c-PAA, indicating that both PAA and TA-c-PAA binders are electrochemically inert substances.

Figure 3. (a) FTIR spectra of PAA, TA and TA-c-PAA. (b) 1H NMR spectrum of TA and TA-c-PAA. (c) DSC plots of PAA and TA-c-PAA. (d) CV testing results of copper foil, Cu@TA-c-PAA and Cu@PAA
From the stress-strain curve in Figure 4(a), the stress-strain curve of the PAA film is linear, and its tensile strength and elongation at break are 9.97 MPa and 5.02% respectively. In contrast, the TA-c-PAA film has better ductility, with a tensile strength of 5.74 MPa and an elongation of 264.51% (shown in Figure 4(d), therefore, the introduction of TA allows the binder to disperse the stress generated during the stretching process. The author then conducted a 180° peeling test on Si@PAA and Si@TA-c-PAA to quantitatively evaluate the adhesion strength between Si electrodes and Cu current collectors, the results are shown in Figure 4(b) and (e) respectively. The average force values of TA-c-PAA and PAA are 2.06 N and 0.51 N respectively, indicating that the bonding strength of TA-c-PAA binder is stronger after TA is added, this can be attributed to the existence of abundant hydrogen bonds in the TA-c-PAA molecular chain. Therefore, the TA-c-PAA binder greatly enhanced the interface interaction and adhesion between the Si electrode film and the copper foil.
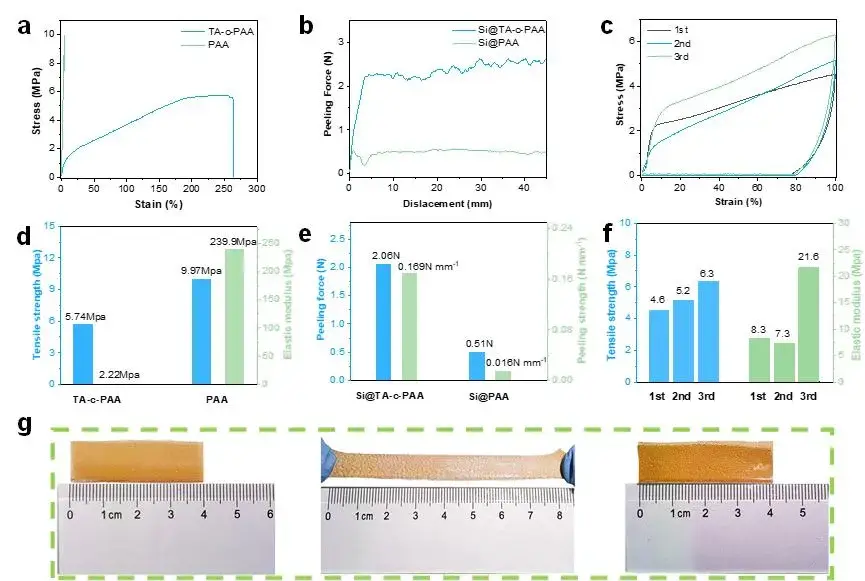
Figure 4. (a) Stress-strain curve of PAA and TA-c-PAA bonded films. (b) 180° stripping curves of Si@PAA and Si@TA-c-PAA electrodes. (c) Cyclic stretching curve of TA-c-PAA adhesive film. (d) Tensile strength and elastic modulus of PAA and TA-c-PAA bonded films. (e) Peeling force and strength of Si@PAA and Si@TA-c-PAA electrodes. (f) Elastic modulus, tensile strength, and maximum force of TA-c-PAA binder during cyclic stretching. (g) Photos of the TA-c-PAA film before and after stretching and shrinkage
In Figure 5, the author used CR2032 type buckle to conduct research on the electrochemical properties of different binders. As can be seen from Figure 5(a), the CV curve of Si@TA-c-PAA gradually increases in intensity as the number of cycles increases, indicating that its electrochemical kinetics has been significantly improved. At the same time, the discharge/charge curves of the Si@TA-c-PAA electrode after 20, 100, 200, 300 and 400 cycles were in good agreement, indicating good reversibility (as shown in Figure 5(b)). In contrast, the reproducibility of the discharge/charge curve of the Si@PAA electrode is poor (as shown in Figure S8). Furthermore, as shown in Figure 5(c) and (d), the Si@TA-c-PAA electrode exhibits stable rate performance under various current densities, for example, the electrode can achieve a high capacity of 1599 mAh/g at a high current density of 2C. In contrast, the Si@PAA electrode only provides a low capacity of 1118 mAh/g at the same current density.
![(a) CV curve of Si@TA-c-PAA electrode at a scanning rate of 0.1 mV s-1. (b) Charge-discharge curve of Si@TA-c-PAA electrode at current density of 0.25C. (c) Rate performance test of Si@TA-c-PAA and Si@PAA electrodes (replicated three times for each binder). (d) Charge-discharge curves at different current densities. (e) Cycling performance of Si@PAA and Si@TA-c-PAA electrodes at 1 C rate (three replicates for each binder). (f) Comparison of cycle performance of silicon-based electrodes under different polymer binders reported in other literature [40,47-50]. (g) Long-term cycling performance of Si@PAA and Si@TA-c-PAA electrodes at a rate of 0.25 C (repeated three times for each binder). Figure 5. (a) CV curve of Si@TA-c-PAA electrode at a scanning rate of 0.1 mV s-1. (b) Charge-discharge curve of Si@TA-c-PAA electrode at current density of 0.25C. (c) Rate performance test of Si@TA-c-PAA and Si@PAA electrodes (replicated three times for each binder). (d) Charge-discharge curves at different current densities. (e) Cycling performance of Si@PAA and Si@TA-c-PAA electrodes at 1 C rate (three replicates for each binder). (f) Comparison of cycle performance of silicon-based electrodes under different polymer binders reported in other literature [40,47-50]. (g) Long-term cycling performance of Si@PAA and Si@TA-c-PAA electrodes at a rate of 0.25 C (repeated three times for each binder).](https://iestbattery.com/wp-content/uploads/2024/05/Figure-5.-aCV-curve-of-Si@TA-c-PAA-electrode-at-a-scanning-rate-of-0.1-mV-s-1.-bCharge-discharge-curve-of-Si@TA-c-PAA-electrode-at-current-density-of-0.25C.webp)
Figure 5. (a) CV curve of Si@TA-c-PAA electrode at a scanning rate of 0.1 mV s-1. (b) Charge-discharge curve of Si@TA-c-PAA electrode at current density of 0.25C. (c) Rate performance test of Si@TA-c-PAA and Si@PAA electrodes (replicated three times for each binder). (d) Charge-discharge curves at different current densities. (e) Cycling performance of Si@PAA and Si@TA-c-PAA electrodes at 1 C rate (three replicates for each binder). (f) Comparison of cycle performance of silicon-based electrodes under different polymer binders reported in other literature [40,47-50]. (g) Long-term cycling performance of Si@PAA and Si@TA-c-PAA electrodes at a rate of 0.25 C (repeated three times for each binder).
At the same time, the authors used electrochemical impedance spectroscopy (EIS) to further study the electrochemical kinetic properties of Si@PAA and Si@TA-c-PAA electrodes, as shown in Figure 6(a). By calculating the Li+ diffusion coefficient, we can see that the Si@TA-c-PAA electrode has a higher Li+ diffusion coefficient than the Si@PAA electrode (as shown in Figure 6(b)). At the same time, using the constant current intermittent titration technology (GITT), it can also be seen that the Si@TA-c-PAA electrode has a high diffusion coefficient during the charge and discharge process, it shows that the TA-c-PAA binder has a good effect in enhancing Li+ diffusion, which is mainly due to the strong self-repairing ability of the binder ensuring the structural stability of the silicon anode.
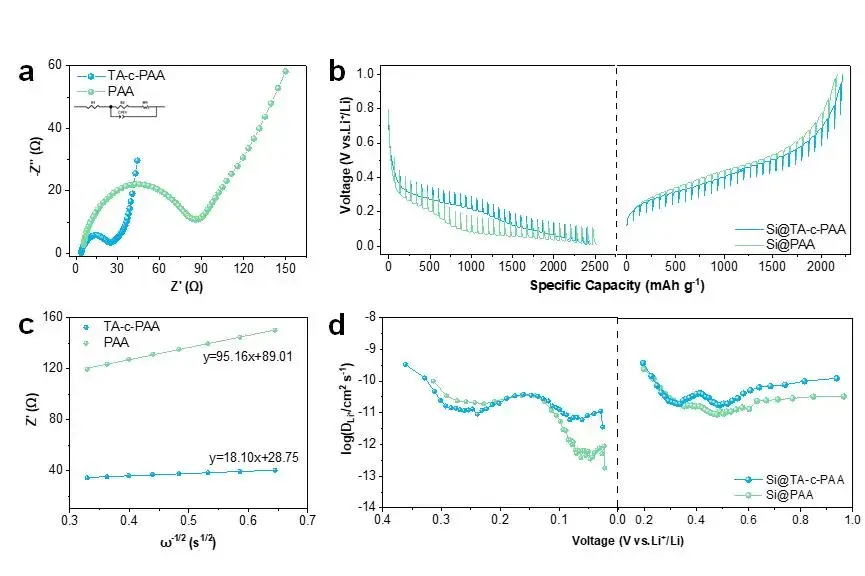
Figure 6. (a) Nyquist plot of EIS pattern. (b) GITT curves of the Si@TA-c-PAA and Si@PAA electrode. (c) The relationship between Z and ω-1/2 for Si@TA-c-PAA and Si@PAA electrodes in the low frequency region. (d) The diffusion coefficient of lithium ions in Si@TA-c-PAA and Si@PAA electrodes.
To further investigate the reasons behind the improved performance of the TA-c-PAA binder, the authors used SEM to study the structural evolution of the Si@TA-c-PAA electrode before cycling and after 100 cycles, as shown in Figure 7(a-h) . For fresh electrodes, we observed tiny original cracks in both Si@PAA and Si@TA-c-PAA electrodes, which may be caused by the high surface tension of the electrodes during the rapid drying process. When the electrode was cycled 100 times, large cracks with a width of 1~2 μm appeared on the Si@PAA electrode, resulting in loss of conductive paths and rapid capacity attenuation. In contrast, the Si@TA-c-PAA electrode still remains dense with only a few tiny cracks, indicating its mechanical structure is strong. The microstructural differences between the two electrodes after cycling indicate that the abundant hydrogen bonds in the TA-c-PAA binder give it unique self-healing properties and flexibility, which can quickly dissipate the stress of the Si particles during the charge and discharge process, this prevents the particles of the Si negative electrode from pulverizing.
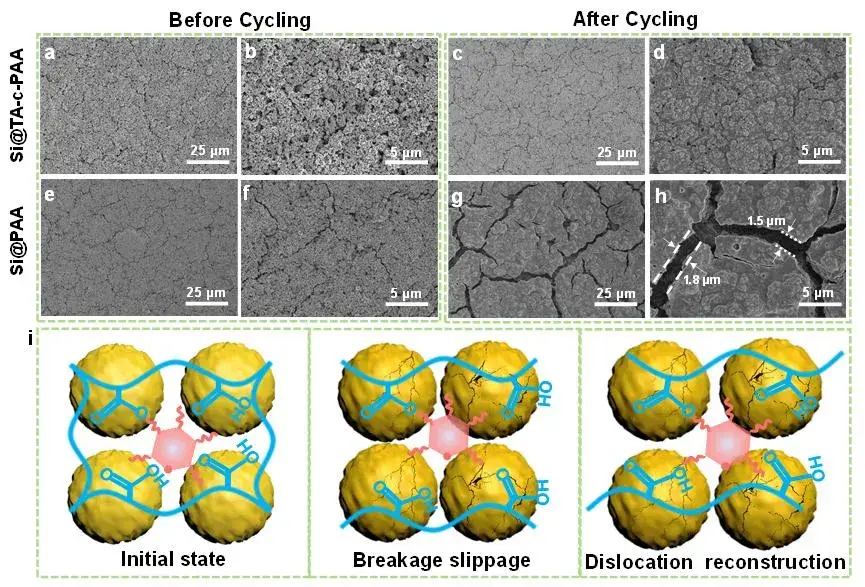
Figure 7. SEM images of (a)-(d) Si@TA-c-PAA electrode and (e)-(h) Si@PAA electrode before and after cycling for 100 times at 0.25C. (i) The self-healing chemical process of cross-linked TA-c-PAA binder with dynamic hydrogen bonding during charge and discharge processes is proposed.
In Figure 8, the authors performed in-situ thickness expansion tests to examine the effects of various binders on suppressing the volume expansion of the silicon anode during charge and discharge cycles. The structure of the in-situ thickness expansion test device is shown in Figure 8(b), in which the force spring and the thickness sensor are assembled in an in-situ expansion analysis system, while the positive electrode, separator and negative electrode are placed between the upper and lower pressure heads. The quality of the NCM523 cathode matches the capacity of the Si@PAA and Si@TA-c-PAA cathodes, and charges and discharges at a 0.1C rate in the voltage range of 3.0-4.25 V, at the same time, the thickness sensor is used to record the thickness changes during charge and discharge in real time. In Figure 8(c), the Si@TA-c-PAA electrode shows a thickness change rate of 82.5% during the lithiation process in the first cycle. In contrast, the thickness change rate of the Si@PAA electrode is 127.1%. During the subsequent delithiation process, the Si@PAA and Si@TA-c-PAA electrodes showed irreversible thickness expansion of 37.5% and 15%, respectively. Overall, the Si@TA-c-PAA electrode exhibited smaller thickness changes and irreversible expansion rates in all cycles, it is shown that dynamic hydrogen bonding promotes the structural stability of silicon-based electrodes (shown in Figure 8(d-e)).
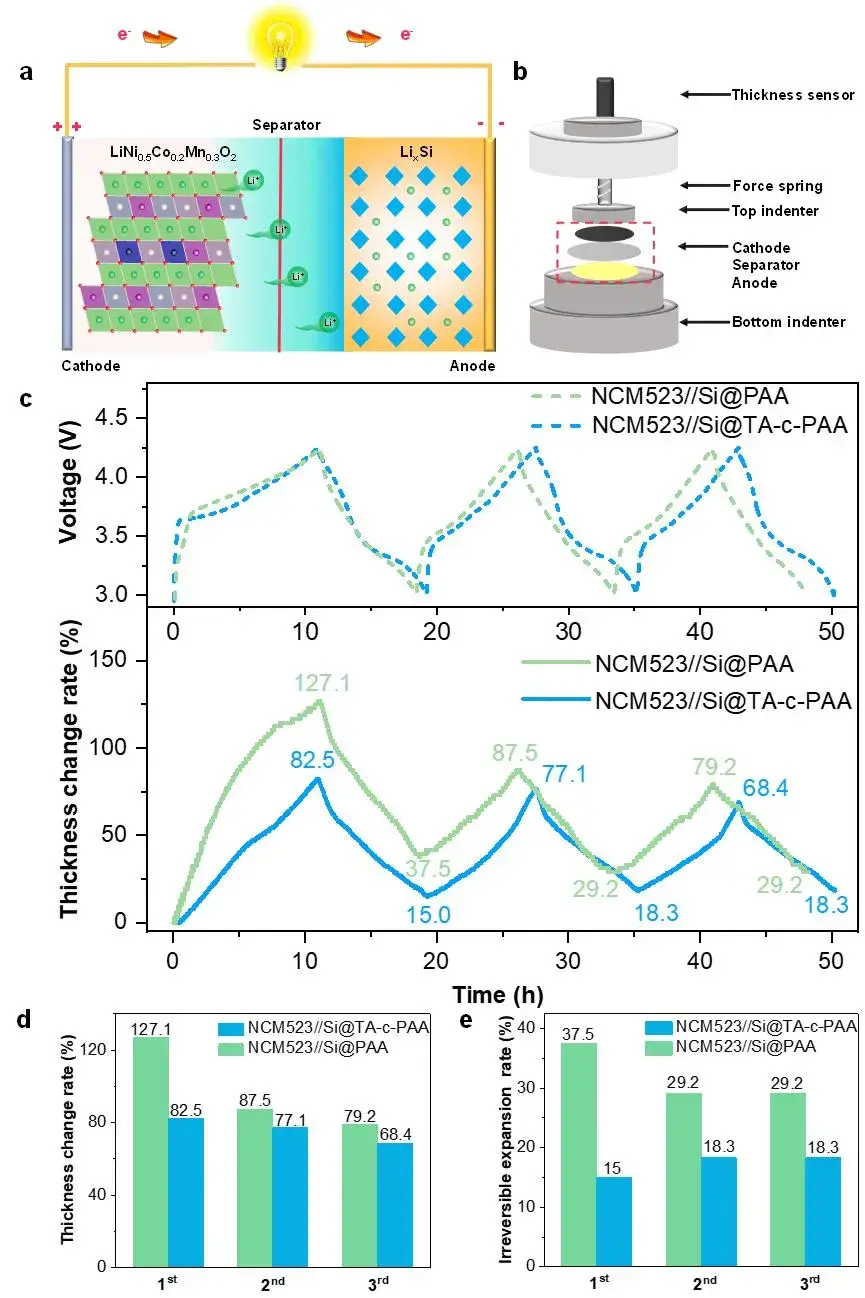
Figure 8.(a) Principle of NCM523//Si full battery. (b) Structural schematic of the in-situ thickness expansion test device. (c) In situ variation curve of thickness of NCM523//Si cell with voltage. (d) Comparison of changes in electrode thickness during cycles. (e) Comparison of the thickness changes of irreversible expansion of electrodes during cycling.
In Figure 9, the author used the COMSOL software to study the relationship between the binder structure and stress dissipation, and conducted finite element simulations of different lithiation states. In the simulation model, the silicon particles are assumed to be independent regular spheres, and they are uniformly distributed in the polymer binder network. Due to the large volume expansion during the lithium intercalation process, there is severe stress concentration on the Si@PAA electrode surface, which may lead to structural failure of the silicon anode. When the maximum stress reached 950 MPa, the silicon particles were broken and crushed (as shown in Figure 9(a)). In contrast, the stress distribution of the Si@TA-c-PAA electrode is lower and its mechanical properties are better (as shown in Figure 9(b)). Therefore, the finite element simulation results show that the TA-c-PAA binder plays an important role in the stress dissipation during the periodic lithium insertion/delithiation process of the silicon anode.
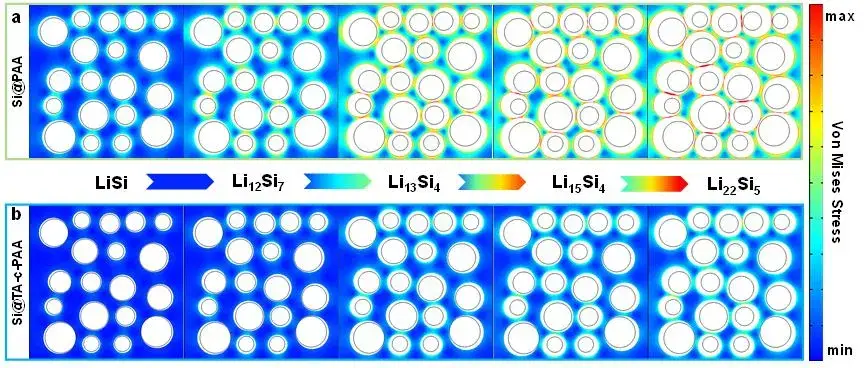
Figure 9. Stress evolution of (a) Si@PAA electrode and (b) Si@TA-c-PAA electrode under different lithiation states.
3. Summary
In this paper, the authors designed and prepared for the first time TA-c-PAA binder for silicon anode. Compared with reported or commonly used binders (e.g., alginate and polyacrylic acid), the TA-c-PAA binder has excellent mechanical properties and imparts excellent electrochemical performance for Si@TA-c-PAA anode. This is due to the fact that the optimized TA-c-PAA binder (1:9) is rich in dynamic hydrogen bonding due to and gives it excellent tensile strength, elongation and flexibility. The corresponding Si@TA-c-PAA electrode also exhibits enhanced interfacial interactions and effective adhesion between the silicon anode electrode and the copper foil, and results in a Si@TA-c-PAA electrode with a high reversible specific capacity (3,250 mAh/g at 0.05C magnification), an excellent multiplication capability (1,599 mAh/g at 2C magnification), and impressive impressive cycling stability (specific capacity of 1742 mAh/g at 0.25C magnification after 450 cycles).
In addition, after non-in situ morphological characterization, in situ swelling thickness analysis and finite element simulation, the authors found that the TA-c-PAA binder can effectively dissipate the stress concentration in the silicon anode during the de-embedded lithium process and prevent the particles from chalking. The mechanism behind this may be related to the self-healing process of TA-c-PAA binder on the microscopic scale. This paper explores the polymer binder by physical cross-linking with TA molecules may provide a generalized solution for efficient and economical binder preparation for silicon anode.
4. Original Text of the Literature
J.H. Chen, Y.X. Li, X.Y. Wu, H.H. Min, J. Wang, X.M. Liu* and H. Yang*. Dynamic hydrogen bond cross-linking binder with self-healing chemistry enables high-performance silicon anode in lithium-ion batteries. Journal of Colloid And Interface Science 657 (2024) 893-902.
5. IEST Related Test Instrument Recommendations:
IEST In-Situ Silicon-Based Anode Swelling Rapid Screening System(RSS1400)
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


