-
iestinstrument
Analyzing the Impact of Slurry Dispersion on Battery Capacity Performance
1. Abstract
In lithium-ion battery electrode manufacturing, achieving a homogeneous slurry dispersion is often considered critical for optimal performance. However, a 2019 study by Kentaro Kuratani and colleagues presents a more nuanced picture. Their research, which correlated battery slurry viscosity with electrode microstructure and cell performance. Surprisingly, the most highly dispersed slurry produced the lowest discharge capacity at high current densities. Cross-sectional CT and electrochemical data indicate that excessive particle breakup can disrupt the conductive-carbon network, reduce electronic percolation in the electrode, and therefore increase polarization under high-rate discharge. These results illustrate that optimal slurry dispersion is a balance between particle deagglomeration and preservation of conductive pathways.
2. Experimental Setup
2.1 Slurry Preparation and Mixing Methods
The investigation employed three distinct mixing procedures to create slurries with varying degrees of particle agglomeration and dispersion, as illustrated in Figure 1
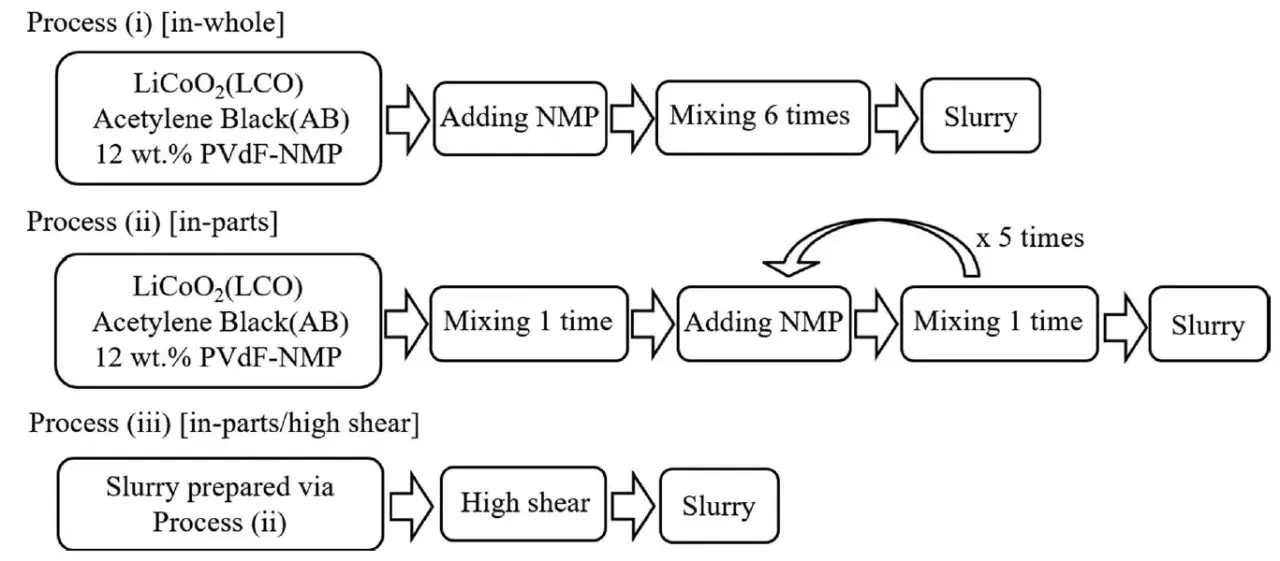
Figure 1. Schematic of the three slurry preparation processes.
2.2 Characterization Techniques
The analysis combined multiple characterization methods:
-
Viscosity Measurement: To assess the rheological behavior of the slurries.
-
Electrode CT Imaging: A non-destructive technique to visualize the 3D microstructure of the coated electrodes, specifically identifying LCO particle agglomerates (shown in blue) within the pore space, as detailed in Figure 2.
-
Coin Cell Rate Capability: Electrochemical testing to evaluate capacity retention under increasing discharge current densities.
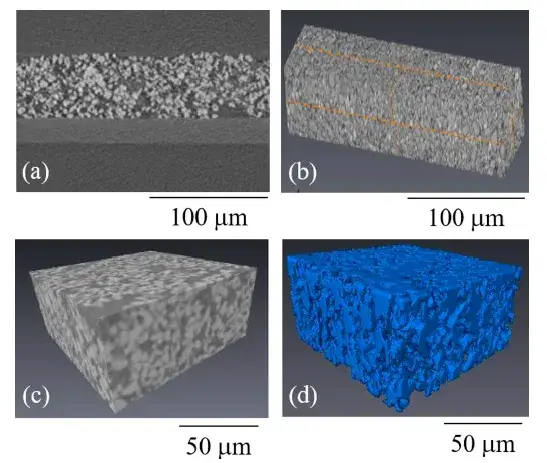
Figure 2. Steps for obtaining a 3D CT reconstruction of the electrode.
4. Analysis of Results
4.1 Battery slurry viscosity: rheology results and interpretation
We measured battery slurry viscosity as a function of shear rate from <1 s⁻¹ up to 100 s⁻¹. At very low shear rates (<1 s⁻¹) all three mixing methods produced near-identical viscosity curves, indicating similar low-shear microstructures. However, in the intermediate shear window (≈1–100 s⁻¹) the viscosity decline patterns diverged:
-
in-whole: showed a modest shear-thinning trend.
-
in-parts: exhibited a steeper viscosity drop than in-whole.
-
in-part + high-shear: displayed the strongest shear thinning across 1–100 s⁻¹.
We interpret these differences as reflecting distinct particle dispersion states and agglomerate breakup induced by the different mixing sequences and energy inputs. Notably, the in-part + high-shear slurry’s sharper viscosity decline suggests a higher fraction of small, well-dispersed primary particles under shear.
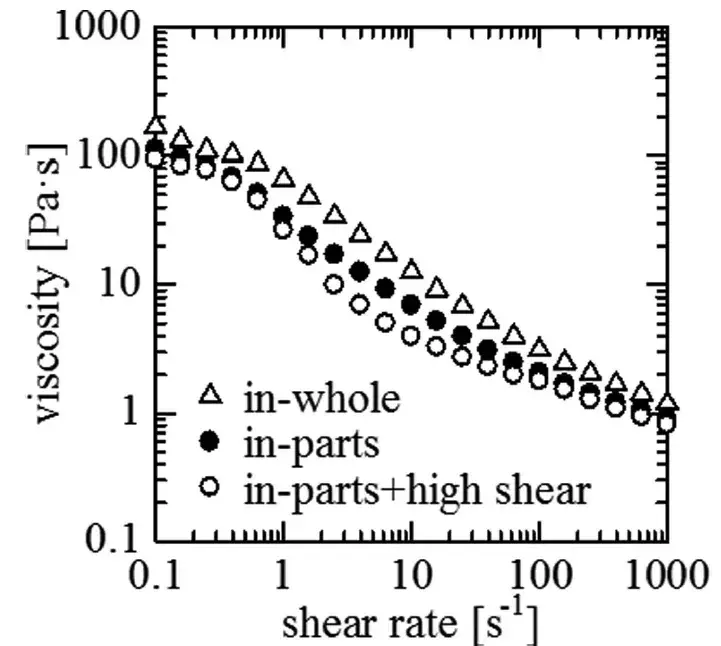
Figure 3. Battery slurry viscosity profiles for three mixing processes
4.2 Electrode CT Imaging
CT imaging provided direct visual evidence of the dispersion states (Figure 4). Electrodes from the ‘in-whole’ process showed numerous large agglomerates. The ‘in-parts’ method significantly reduced this agglomeration. Notably, the ‘in-part+high shear’ electrodes appeared virtually free of large agglomerates, confirming it produced the highest degree of slurry dispersion.
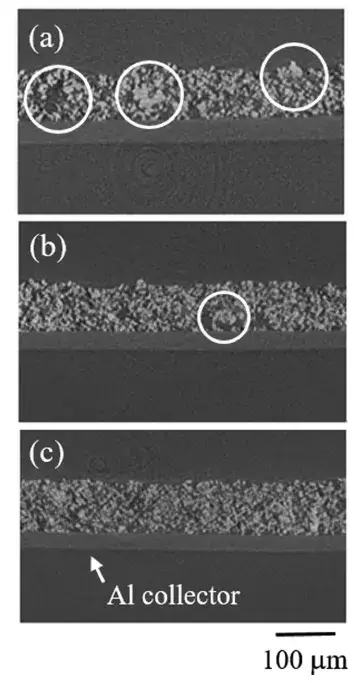
Figure 4. Electrode CT imaging with three stirring methods
4.3 Electrochemical Rate Performance
The rate capability data revealed a critical trend (Figure 5). While performance was similar at low current densities, a stark divergence occurred at a high rate of 1000 mA/g. The ‘in-part+high shear’ cell, despite its superior dispersion, suffered a significantly larger capacity loss. Its charge-discharge curves also showed a larger voltage polarization.
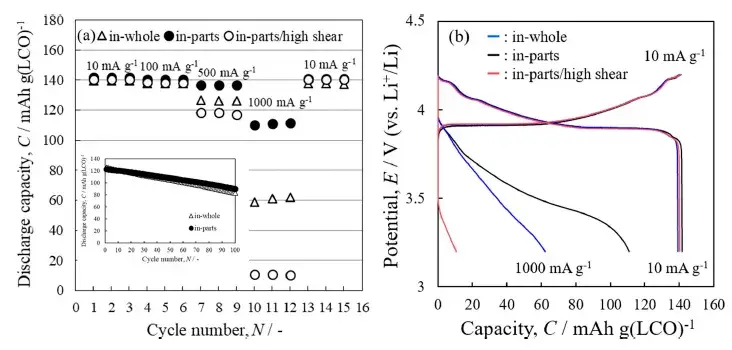
Figure 5. Buckling multiplier performance of the three stirring methods
4.4 Correlating Dispersion State with Electronic Conductivity
The combined data provides a clear narrative. Superior particle dispersion, while minimizing agglomerates, can inadvertently disrupt the percolating network of conductive carbon. This is illustrated schematically in Figure 6. The resulting decrease in electronic conductivity within the electrode leads to increased polarization under high current loads, ultimately diminishing capacity. When the cells were returned to lower current densities, their capacities recovered, confirming the issue was related to kinetics (electronic transport) rather than permanent damage.
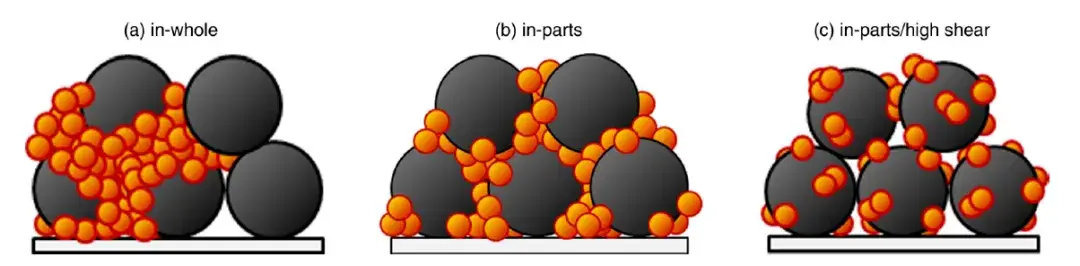
Figure 6. Schematic of particle dispersion for three mixing methods
5. Mechanistic discussion: why better slurry dispersion reduced high-rate capacity
Combining CT and electrochemical results supports the following mechanism without speculation:
-
High dispersion → particle isolation. The in-part + high-shear protocol produced a finely dispersed particle distribution that minimized large agglomerates.
-
Disruption of conductive-carbon network. Fewer particle clusters reduced the opportunities for conductive-carbon bridges to form continuous electronic pathways across the electrode. In effect, the percolation network of conductive carbon became less connected.
-
Increased electronic resistance → higher polarization at high rates. With poorer electronic percolation, the electrode’s effective electronic conductivity decreased. Consequently, at high current densities the electrode exhibited larger polarization and reduced usable capacity.
-
Reversibility at low rates. At low current densities, ionic limitations dominate less and electronic contact sufficed to recover capacity, which explains the return to similar capacities when the current was lowered.
This mechanism explains the counterintuitive finding: maximum particle dispersion does not guarantee maximum rate capacity — conductive network integrity is equally important for high-power performance.
6. Summary
This study demonstrates that the relationship between slurry dispersion and electrochemical performance is not linear. Optimizing the mixing process involves striking a careful balance. Excessive dispersion can be detrimental to high-rate capacity by compromising the electronic conductive network within the electrode. Therefore, comprehensive characterization, combining rheology (battery slurry viscosity), microstructure analysis (CT), and electrochemical testing, is essential for identifying the optimal electrode manufacturing parameters.
7. References
Kentaro Kuratani, Kaoru Ishibashi, Yoshiyuki Komoda, Ruri Hidema, Hiroshi Suzuki and Hironori Kobayashi1. Controlling of Dispersion State of Particles in Slurry and Electrochemical Properties of Electrodes. Journal of the Electrochemical Society, 166 (4) A501-A506 (2019)
8. Recommended Test Equipment: IEST Slurry Resistance Analyzer(BSR Series)
For researchers conducting similar analyses, the BSR Series Slurry Resistance Analyzer from IEST is designed for precise characterization of slurry properties.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.




