-
iestinstrument
How Does Conductive Additives Content Affect Lithium Battery Performance? Experimental Data Reveals the Optimal Ratio!
1. Introduction: Conductive Additives—The “Invisible Bridge” in Lithium Batteries
In lithium battery electrodes, conductive additives and binders are mixed to form a continuous carbon-binder domain (CBD), with active material particles embedded within this network. The CBD serves as the primary pathway for electron and ion transport. On one hand, conductive additives establish an interconnected three-dimensional network to conduct electrons, akin to neural networks in the human body. On the other hand, the CBD contains submicron- and nano-scale pores filled with electrolyte, facilitating lithium-ion diffusion, much like capillaries. This CBD directly impacts the efficiency of electron/ion transport and overall battery performance. Conductive additives act as the “bridge” that connects active material particles, reducing resistance and enhancing rate capability. However, different conductive additives create distinct pore microstructures, influencing ion diffusion coefficients. Yet, this “bridge” is not always better with higher content—insufficient additives lead to broken electron pathways, while excessive additives cause slurry dispersion issues, process instability, and cost escalation.
The distribution state of conductive additives in the electrode is influenced not only by factors such as the type and morphology of the additives but also by the processing conditions. For example, whether the slurry dispersion achieves a uniform distribution of the conductive additives, whether the drying process avoids stratification and maintains an optimal distribution in the slurry, and whether the calendaring process facilitates the formation of interconnected conductive pathways. Therefore, the design and manufacturing process of the electrode is a complex undertaking.

Figure 1. Conductive additives as critical auxiliary materials in lithium batteries
Recently, we conducted experiments examining the correlation between the slurry resistance and electrode resistance at different conductive additive loadings to address a classic challenge in the industry: How to determine the “sweet spot” for additive content that optimally balances battery performance, processing stability, and cost. In practical manufacturing, the addition of conductive additives often faces a trade-off: from a technical perspective, insufficient additive results in high electrode resistance, which can lead to excessive heating and capacity fade during fast charging; conversely, excessive additive increases slurry viscosity, leading to coating defects such as cracking and powder shedding. From a cost perspective, conductive additives (e.g., conductive carbon black, carbon nanotubes) are expensive, and higher loadings directly increase the cost per ton of slurry. To address these issues, we designed a gradient experiment and performed quantitative analyses to provide a scientific basis for additive incorporation.
2. Experimental Design: Multi-Dimensional Validation Across Five Gradients
2.1 Sample Preparation
Variables were strictly controlled. The composition (and ratio) of materials was fixed—using a cathode active material (LCO), binder (PVDF), and solvent (NMP)—with only the conductive additive (SP) content varied. Five gradients were set at 0.5%, 1.0%, 1.3%, 1.5%, and 1.8%, while maintaining consistent processing conditions.
2.2 Testing Equipment and Methods
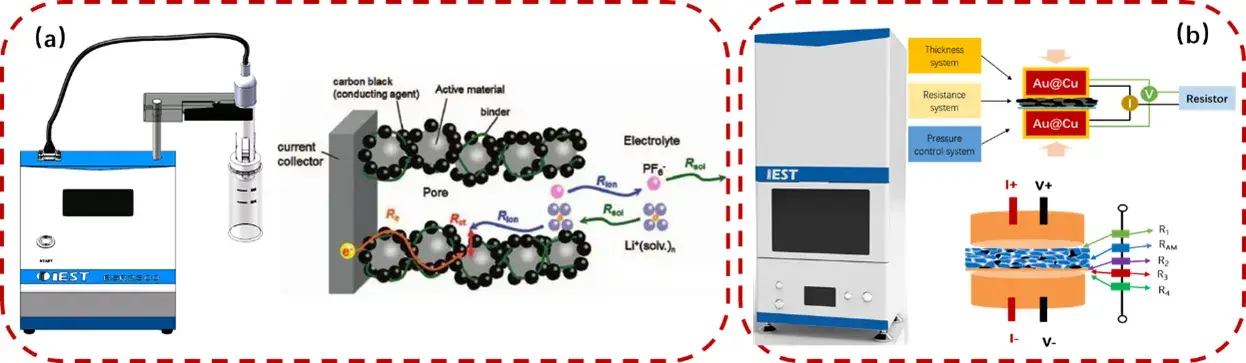
A combination of the IEST BSR series for slurry resistance measurements and the IEST BER series for electrode resistance measurements was used to analyze samples with different conductive additive contents.
Figure 2. Schematic of the Slurry Resistance Tester(BSR Series) and Electrode Resistance Testing Equipment(BER Series)
3. Experimental Results: The “Performance Inflection Point” of Conductive Additives
The ideal distribution state of the conductive additive in the slurry is one in which the particles are uniformly dispersed and interact strongly with the active material (forming a coating structure), while simultaneously establishing an interconnected network among themselves. This ideal state must also be maintained throughout subsequent processing. Figure 3 illustrates the test results showing the variation of slurry resistivity with increasing conductive additive content. The experimental data indicate that the conductive additive content has a significant impact on slurry resistance, processability, and cost, and a clear “critical threshold” exists. As the conductive additive content increases from 0.5% to 1.5%, slurry resistivity exhibits a steep decline. This rapid reduction is mainly attributed to the gradual formation of a continuous conductive network by the additive particles, which provides an efficient electron transport pathway. However, when the additive content exceeds 1.5%, the resistance tends to level off; indeed, a slight increase in resistivity is observed when the content is raised from 1.5% to 1.8%. This is likely due to localized agglomeration—resulting in the formation of “islands” of conductive additive—that in turn impedes electron transfer between active material particles. This phenomenon demonstrates that the effectiveness of the conductive additive has a distinct “saturation point,” beyond which performance improvements become negligible and may even be counterproductive due to dispersion issues.
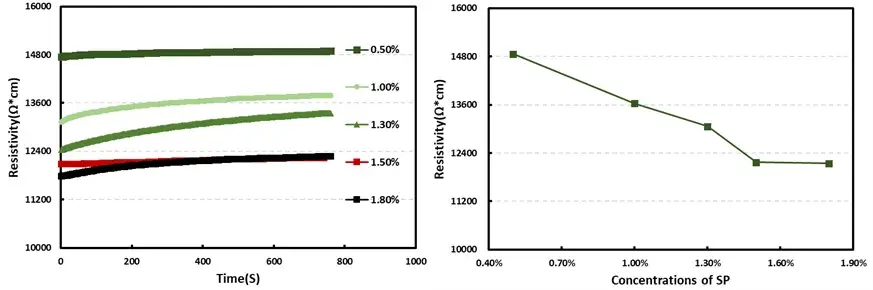
Figure 3. Variation in Slurry Resistivity with Increasing Conductive Additive Content
In addition to changes in resistivity, the conductive additive content also significantly affects the slurry’s processability and production cost. Excessive additive increases the viscosity, which can lead to poor leveling during the coating process and result in uneven electrode thickness. This is manifested as a “fish-scale” defect on the electrode surface, directly affecting capacity uniformity.
Compared to the effect on the slurry-level resistivity, the influence of conductive additives on electrode-level resistance is of greater concern. Moreover, assessing the correlation between slurry resistance and electrode resistance can help identify abnormal process stages early, thereby preventing wasted time and resources. In our experiment, we further evaluated the impact of different conductive additive levels on electrode resistance. As shown in Figure 4, the electrode resistivity varies with the additive content. For each level of additive content, six different positions on the electrode were measured to calculate the mean resistivity and coefficient of variation (COV). The data clearly demonstrate that the conductive additive content has a significant effect on electrode resistivity. At 0.5% additive content, the testing equipment even recorded values beyond the measurable range. As the content increases from 1.0% to 1.5%, electrode resistivity decreases dramatically—from 23,604.99 Ω·cm to 299.52 Ω·cm—displaying a reduction by several orders of magnitude. With further increases in additive content, the electrode resistivity shows a slight rebound, mirroring the behavior observed in slurry resistivity, and reinforcing the notion of a “saturation point.” Additionally, the increased COV at 1.8% additive content indicates significantly greater fluctuation in resistance across different electrode regions, further corroborating that the “performance inflection point” may induce localized agglomeration. Overall, resistance evaluation serves as an effective pre-assessment method for determining the appropriate additive content range and validates that conductive additive incorporation should adhere to a “balancing principle” — satisfying the minimum requirements for a conductive network while avoiding performance degradation and excessive cost.
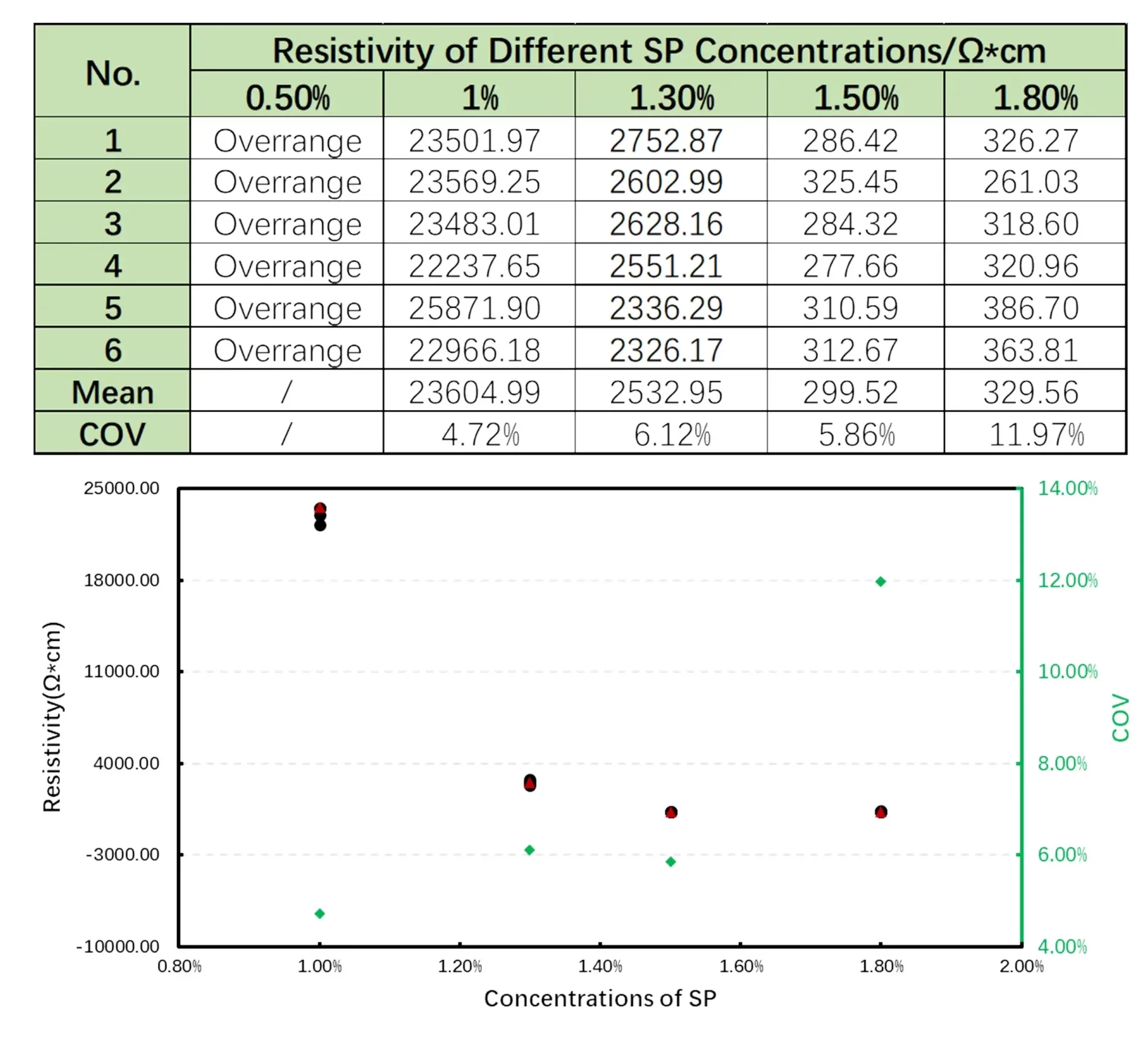
Figure 4. Changes in electrode resistivity as the proportion of conductive additive increases
4. Conclusion: The “Balancing Philosophy” of Conductive Additives
5. References
[1] Ishii M , Makino S , Nakamura H. The role of carboxymethyl cellulose on the rheology of anode slurries in lithium-ion batteries[J]. Current Opinion in Colloid & Interface Science, 2024, 74(000):10.DOI:10.1016/j.cocis.2024.101858.
[2] Jin B , Gu H B , Kim K W. Effect of different conductive additives on charge/discharge properties of LiCoPO4/Li batteries[J].Journal of Solid State Electrochemistry, 2008, 12(2):105-111.DOI:10.1007/s10008-007-0367-4.
[3] Yang L H .Synergetic effect of conductive additives on the performance of high power lithium ion batteries[J].New Carbon Materials, 2012.DOI:10.1016/S1872-5805(12)60026-2.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.