-
iestinstrument
Solid-state Electrolytes Testing Method for Electrochemical Properties
1. Preface
The performance of solid-state electrolytes—especially their ionic conductivity and cycling stability—is highly dependent on physical characteristics such as density, surface roughness, and structural integrity. Achieving consistent and accurate electrolyte measurement is essential for advancing the development of all-solid-state batteries. Reliable solid state test systems must provide stable and quantifiable pressure application throughout testing to ensure reproducible and meaningful results. This underscores the importance of integrated manufacturing and testing platforms capable of delivering standardized mechanical conditions.
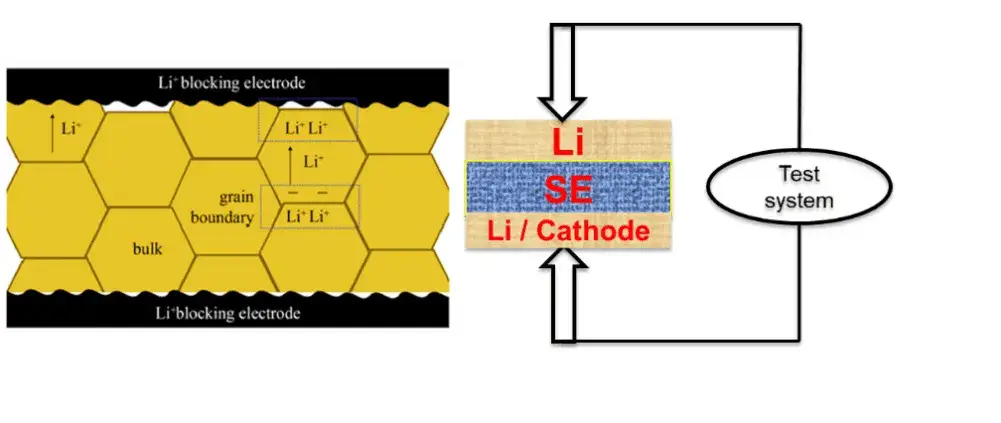
Figure 1. Schematic diagram of the testing of solid state electrolytes
2. Testing Instrument
The Solid-State Electrolyte Test System model SEMS1100, co-developed by IEST and Xiamen University, is an advanced multi-functional platform designed specifically for solid-state electrolyte evaluation. This integrated system combines several key modules:
- Pressurization unit
- Electrochemical testing station
- Density measurement cell
- Ceramic pellet compression and clamping fixtures
It supports a wide range of solid state electrolyte materials, including oxide-based, sulfide-based, and polymer electrolytes. The SEMS1100 enables high-precision electrolyte measurement under controlled pressure, making it ideal for R&D and quality control applications.
Figure 2. Schematic diagram of the solid-state electrolyte test system(SEMS Series) equipment
3. Application Cases
3.1 Powder Production
To perform accurate electrolyte measurement, solid electrolyte powders must be compressed into uniform pellets. Inconsistent pressure during this process often results in cracked or uneven samples. As shown in Figure 3, the SEMS1100 system produces pellets with superior structural integrity and surface uniformity across varying pressure ranges. This improves manufacturing yield and ensures more reliable solid state test outcomes.
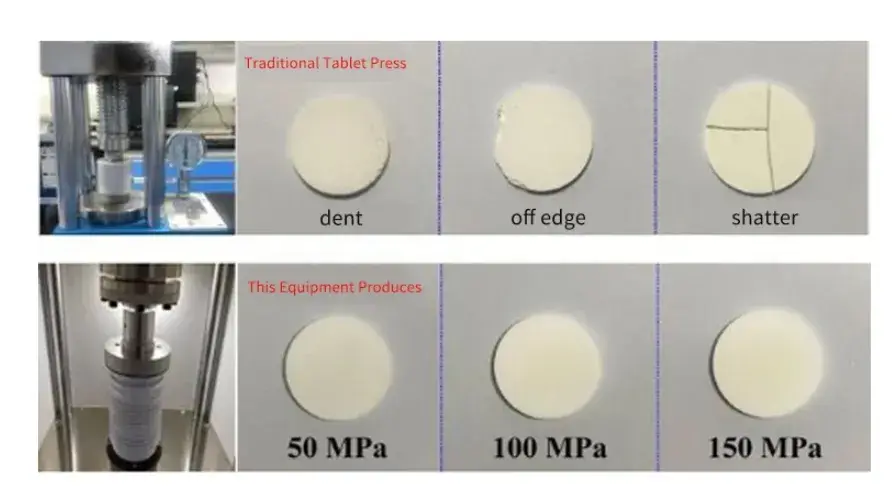
Figure 3. Comparison of the production results of different devices
3.2 Lon Electrical Conductivity
The ionic conductivity of solid electrolytes such as LATP and LLZO is highly sensitive to applied pressure. Using the SEMS1100, electrochemical impedance spectra (EIS) can be captured under precise mechanical conditions. Figure 4 illustrates how quantified pressure application significantly influences ionic conductivity—highlighting the necessity of standardized solid state test protocols for meaningful electrolyte measurement.
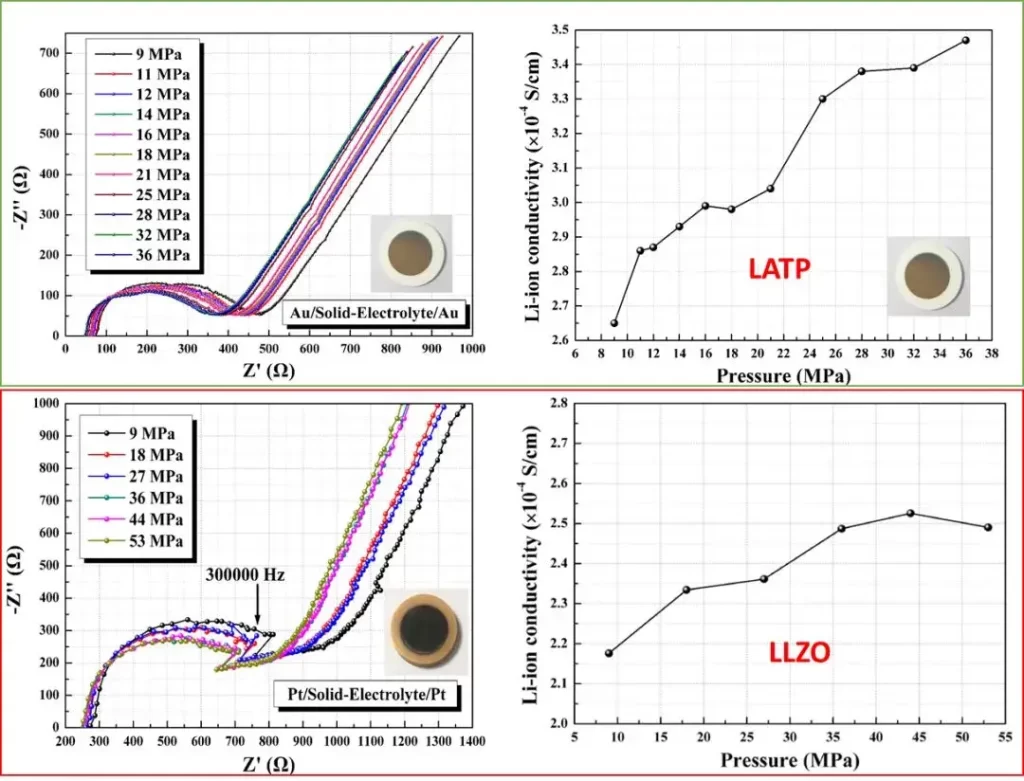
Figure 4. Electrochemical impedance spectra of two solid state electrolytes and their ionic conductivity to pressure
3.3 Electron Conductivity & Compaction Density
With SEMS1100, both electronic conductivity and compaction density of LATP powder can be measured simultaneously. Results show compaction density increases from 1.7 g/cm³ to 2.1 g/cm³ with rising pressure, while electronic conductivity stabilizes around 50 MPa(see Figure 5).
This demonstrates that density and electronic conductivity trends of solid state electrolytes do not always align, emphasizing the need for comprehensive electrolyte measurement under different test conditions.
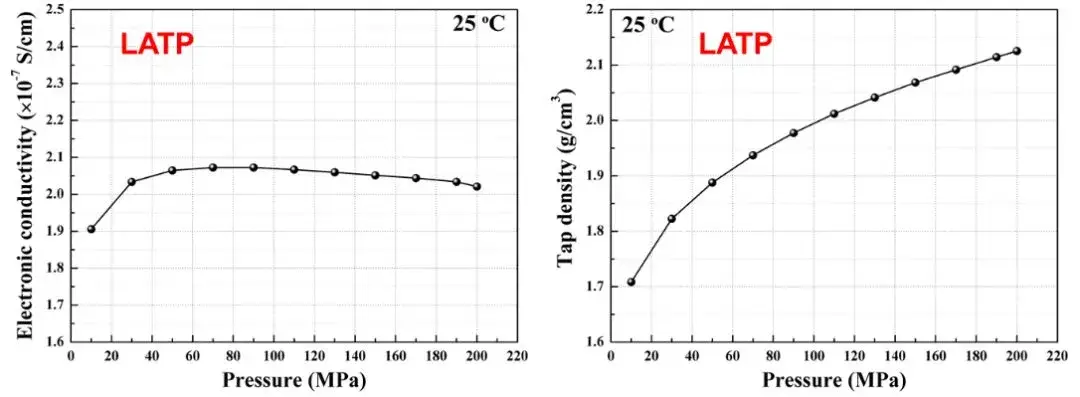
Figure 5. LATP Change of electron conductivity and compaction density of solid state electrolyte
3.4 Recycling Performance of Solid-state Lithium Metal Battery
Using a Li-SE-Li symmetric cell configuration, the influence of pressure on lithium deposition stability was evaluated. A decrease in applied pressure from 120 MPa to 110 MPa resulted in a noticeable increase in overpotential (Figure 6), confirming that interfacial stability is strongly pressure-dependent. This type of solid state test is essential for evaluating long-term battery performance.
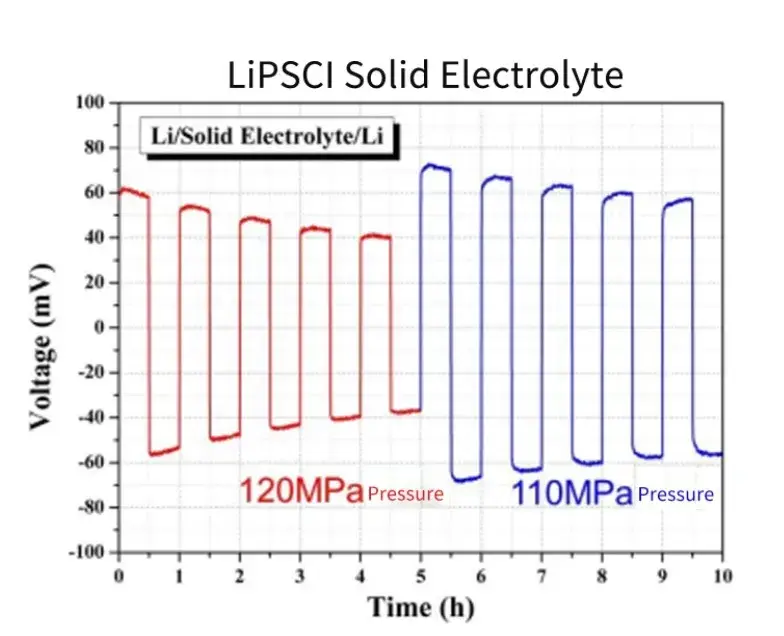
Figure 6. Cycle charge and discharge test of a symmetrical battery
3.5 Electrochemical Stabilization Window
The electrochemical window of a Li-SE-stainless steel battery was evaluated using cyclic voltammetry. Results indicate that the solid state electrolyte remains stable within a voltage window of 0–3V, as oxidative current density remains low (≈1.2 A cm⁻² at 3V).
This confirms SEMS1100’s capability for solid state tests, enabling pressurization and sealing for comprehensive evaluation of electrolyte measurement and electrochemical stability in lithium-metal batteries.
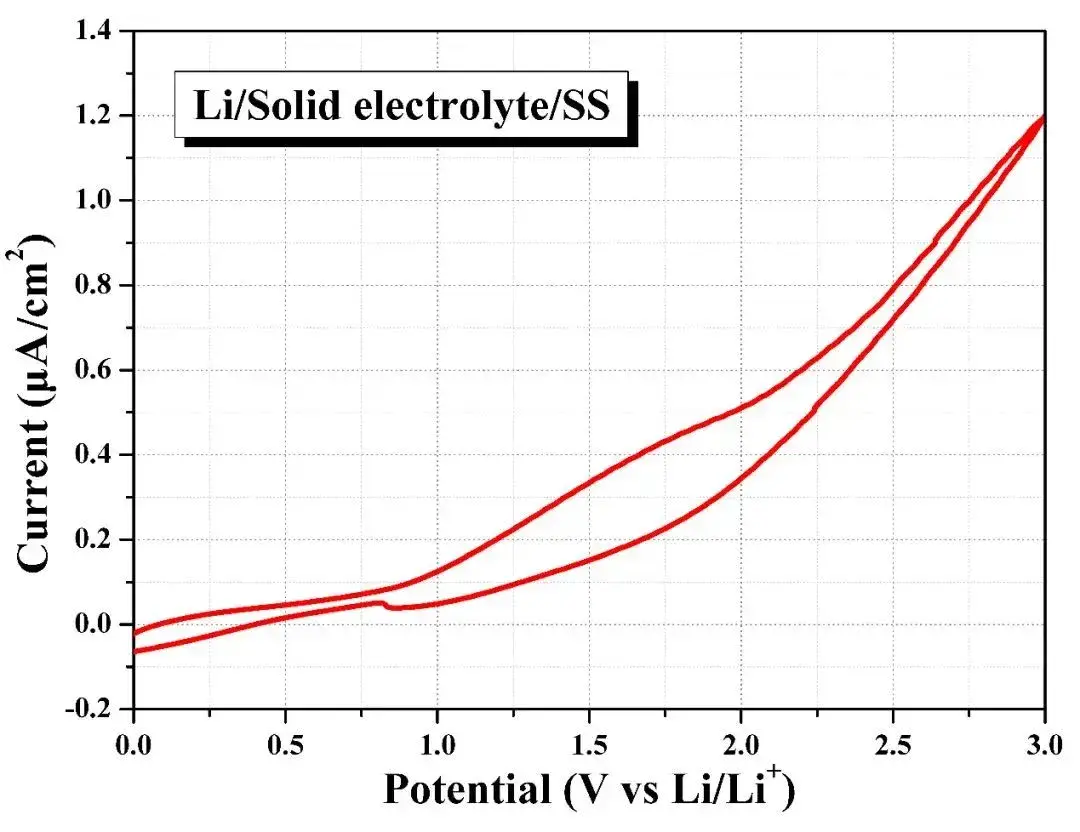
Figure 7. Electrochemical Window Test of Solid state electrolytes
4. Summary
Using the integrated solid state electrolyte test system SEMS1100, researchers can accurately measure:
-
Ionic conductivity
-
Electronic conductivity
-
Compaction density
-
Electrochemical stability window
-
Lithium-metal battery interface stability & cycle performance
These measurements, conducted under controlled pressure conditions, provide reliable insights into the design and development of next-generation solid state electrolytes and solid-state batteries.
By offering standardized and repeatable solid state test procedures, SEMS1100 accelerates electrolyte material research and promotes breakthroughs in battery technology.
5. FAQ on Solid-State Electrolyte Testing
Q1: Why is pressure control important in solid state electrolyte testing?
A: Applied pressure ensures uniform contact between electrolyte particles and electrodes, reducing interfacial resistance and preventing artifacts in electrolyte measurement. Consistent pressure is critical for obtaining reliable and reproducible solid state test results.
Q2: What types of solid-state electrolytes can the SEMS1100 test?
A: The system is compatible with oxide-based (e.g., LATP, LLZO), sulfide-based, and polymer solid state electrolyte materials, supporting a wide range of solid state test applications.
Q3: Can the SEMS1100 measure both ionic and electronic conductivity?
A: Yes, it is equipped to perform simultaneous electrolyte measurement of ionic and electronic conductivity, along with compaction density, in a single pressing cycle.
Q4: How does the system improve the reliability of pellet preparation?
A: The SEMS1100 applies uniform and quantifiable pressure, reducing the risk of pellet cracking or uneven surfaces. This leads to higher yield and more consistent solid state test outcomes.
Q5: Is the SEMS1100 suitable for battery cycling tests?
A: Absolutely. The system supports the assembly and testing of symmetric and asymmetric cells under controlled pressure, allowing evaluation of cycling performance and interfacial stability.
Q6: Can SEMS1100 evaluate lithium-metal batteries?
A: Yes. It enables testing of Li-SE-Li symmetric batteries, measuring deposition behavior, overpotential, and long-term cycling under controlled pressure.
6. References
[1] Huang Xiao, Wu Linbin, Huang Zhen, et al. Characterization and testing of key electrical and electrochemical properties of lithium-ion solid electrolytes, Energy Storage Science and Technology, 2020,9 (2): 479-500.
[2] Yong-Gun Lee, Satoshi Fujiki, Changhoon Jung, et al.High-energy long -cycling all-solid-state lithium metal batteries enabled by silver–carbon composite anodes.2020, 4(5): 299-308.
[3] Nicholas Williard a, Chris Hendricks a, Jaesik Chung, et al. Effects of external pressure on phase stability and diffusion rate in lithium-ion cells.Journal of Electroanalytical Chemistry, 2021, 895, 115400.
[4] T / SPSTS 019- -2021; Performance Requirements and Test Methods of Solid State Electrolytes for Solid State Lithium Battery; Inorganic oxide; Solid State Electrolytes for Solid State Electrolytes.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.