-
iestinstrument
Novel Method to Characterizing Battery Slurry Conductivity and Sedimentation Stability
1. Preface
The electrode slurry is a crucial intermediate product in lithium-ion battery manufacturing. Its homogeneity and stability significantly impact the consistency and electrochemical performance of the final cells. Currently, Battery slurry viscosity is often the sole parameter used for slurry monitoring, which fails to fully assess the uniformity and stability of its electrical properties.
Slurry resistivity, however, offers a more direct insight. This parameter is highly sensitive to the battery slurry formulation, including the types and amounts of active materials, conductive agents, and binders. Furthermore, as slurries rest after mixing, gelation or sedimentation can occur (Figure 1), leading to measurable changes in resistivity. Consequently, monitoring slurry resistivity provides a powerful method for characterizing the electrical homogeneity and stability of battery slurries.
Implementing resistivity monitoring enables material evaluation and screening by assessing the impact of different components on performance. It also serves as a critical process control tool, allowing for the rapid identification of anomalies during the mixing stage. This helps prevent defective batches from progressing downstream, saving both time and cost.
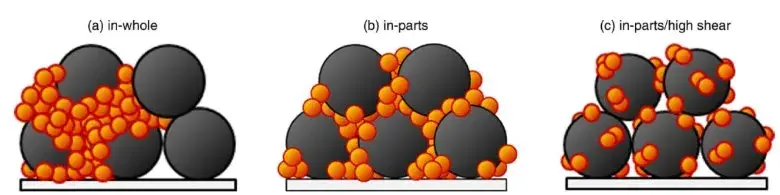
Figure 1. Schematic diagram of three mixing states for LCO and conductive carbon particles.
2. Equipment and Methodology for Slurry Resistivity Testing
2.1 Experimental Equipment
The IEST Slurry Resistance Tester (BSR2300), featuring three independent electrode channels, was used for this analysis.
2.2 Testing Procedure
The slurry sample is placed in a beaker with a diameter greater than 35 mm. The electrode probe is immersed into the slurry and gently agitated to ensure the electrode surface is fully wetted. The accompanying software is then activated to collect real-time resistivity data from each electrode channel.
3. Battery Slurry Resistivity Characterization Case Studies
3.1 Case study A: effect of viscosity on slurry resistivity (battery slurry viscosity)
Viscosity governs particle mobility and contact probability in a slurry. We compared two carbon-conductive slurries with different viscosities while keeping formulation nominally constant. Results show:
-
The higher-viscosity slurry exhibited lower resistivity than the lower-viscosity slurry under identical measurement conditions.
-
This implies that, at higher viscosity, conductive particles form more stable contacts and a more continuous percolation network.
As a practical metric, resistivity measurement can therefore validate whether batches with the same target viscosity maintain comparable conductive connectivity.
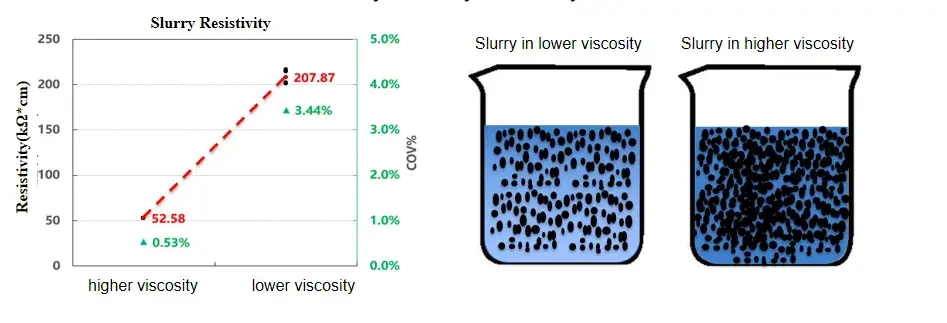
Figure 2. Resistivity comparison of slurries with different viscosities.
3.2 Case study B: Effect of Dilution On Slurry Resistivity (battery slurry formulation)
Diluting the conductive carbon slurry by different multiples can change the concentration of conductive carbon particles per unit volume and affect the dispersion state of conductive carbon particles in the slurry. As the dilution factor increases, the concentration of conductive carbon gradually decreases and the resistivity of the slurry gradually increases. If the slurry formula is fixed in the mass production of slurry, the resistivity test can be performed on different batches of the same concentration slurry monitor the process stability of the slurry.
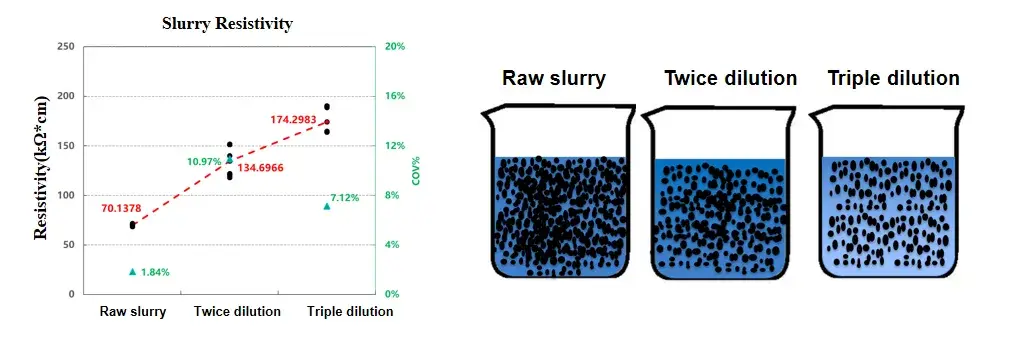
Figure 3. Comparison of resistivity of different dilution multiples of slurry
3.3 Case study C: Sedimentation Performance Monitored by Resistivity (battery slurry viscosity)
We monitored an NCM811 slurry continuously over three days to detect settling. The vertical test channels measured resistivity at distinct heights in the bottle. Notable observations:
-
On the third morning, the lower channel’s resistivity dropped markedly, indicating that particles from the upper layers had settled to the bottom and locally increased conductivity.
-
By tracing resistivity versus time at multiple vertical positions, one can estimate the slurry’s practical “maximum static hold time” (i.e., how long the slurry can sit before significant settling compromises homogeneity).
This time-resolved approach helps production staff decide how long to store mixed slurry before recoating or re-agitation is required, thus reducing process scrap and variability.
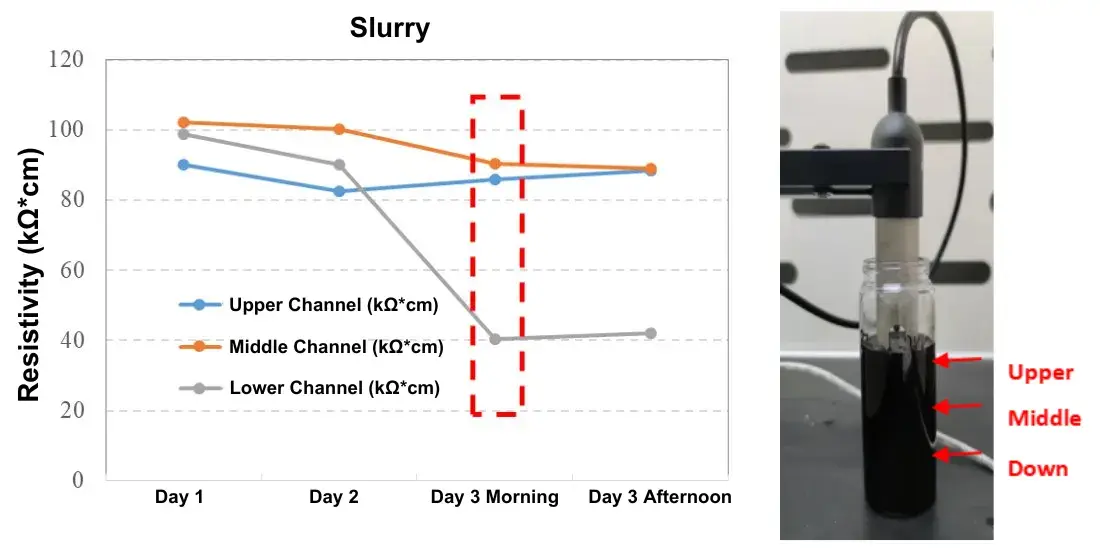
Figure 4. Slurry resistivity change over time, indicating sedimentation.
4. Practical Tips and Measurement Considerations
-
Bottle geometry: Use a standardized container (mouth diameter > 35 mm) to ensure probe immersion repeatability.
-
Probe wetting: Brief stirring to wet the probe is essential to avoid air gaps that artificially increase measured resistivity.
-
Multi-point probing: Use vertical channel spacing to detect incipient sedimentation rather than a single-point measurement.
-
Establish recipe-specific tolerances: Define acceptable resistivity windows for each battery slurry formulation and for different viscosity grades.
-
Combine metrics: Interpret resistivity in the context of battery slurry viscosity measurements and imaging to avoid misinterpreting rheology-induced effects as formulation drift.
5. Summarize
This paper uses a Battery Slurry Resistivity Tester(BSR2300 IEST) to monitor the slurry resistivity with different viscosities, different dilution multiples and different standing times, which can distinguish the differences in the slurry resistivity under different formulations, different processes, and different quality states. By establishing specification limits for slurry resistivity, R&D and production teams can effectively monitor process stability and determine the maximum standing time, ensuring consistent, high-quality electrode manufacturing.
5. References
[1] B.G. Westphal et al. Journal of Energy Storage 11 (2017) 76–85.
[2] Kentaro Kuratani, Kaoru Ishibashi, Yoshiyuki Komoda, Ruri Hidema, Hiroshi Suzuki and Hironori Kobayashi1. Controlling of Dispersion State of Particles in Slurry and Electrochemical Properties of Electrodes. Journal of the Electrochemical Society, 166 (4) A501-A506 (2019)
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.