-
iestinstrument
Analysis the Relationship Between Capacity Decay And Thickness Swelling During The Long-cycle Process Of NCM Cells
Capacity decay and loss will occur during the cycle of lithium-ion batteries, in order to improve battery capacity and performance, scholars at domestic and international have fully studied the mechanism of lithium-ion battery capacity decay. At present, it is known that the main factors that cause the capacity decay of lithium-ion batteries include the formation of CEI/SEI passivation film on the surface of the cathode and anode electrodes, metal lithium deposition, dissolution of electrode active materials, occurrence of redox reactions or side reactions at the cathode and anode, structural changes, and phase changes. Change etc. 1~3. At present, the change of lithium-ion battery capacity decay and its reasons are still in the process of continuous research. In this paper, by studying the stress change and electrochemical behavior of NCM/graphite cells during the cycle process, the reasons for the cell cycle capacity decay are analyzed.
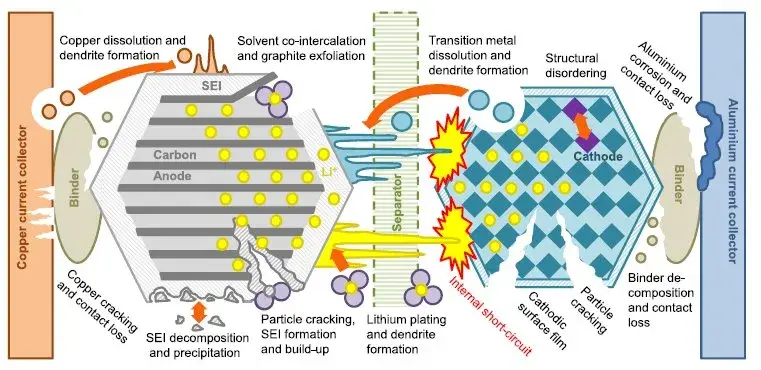
Figure 1. Causes of cell failure
1. Test Information
1.1 Test Equipment
In-situ swelling analyzer, model SWE2110 (IEST), which can apply a pressure range of 50~10000N.
Figure 2. Schematic diagram of in-situ swelling analyzer
2. Testing Parameters
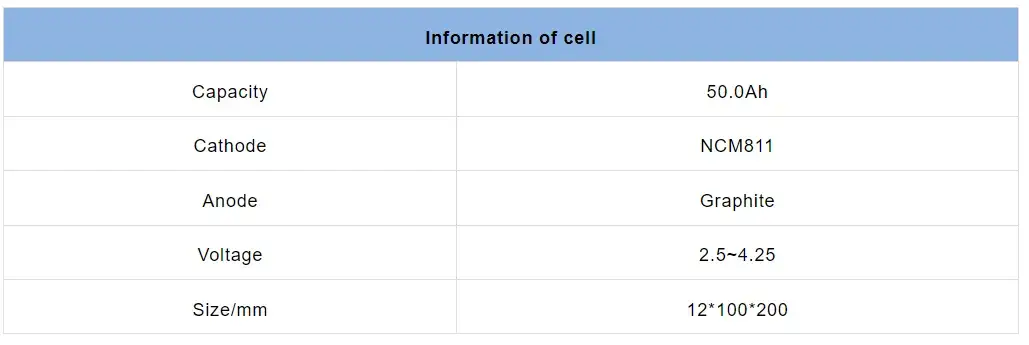
2.1 The cell information is shown in Table 1.
Table 1. Cell Information
2.2 Test Process
Put the two largest surfaces of the cell on cushion pads, place them in the test chamber of the in-situ swelling analyzer, set the charge and discharge parameters: 25°C for 30 minutes; charge 1.0C, cut-off current 0.05C; leave for 30 minutes, discharge 1.0 C. The cut-off voltage is 2.5V. Simultaneously turn on the in-situ swelling analyzer, set the experimental mode (50kg constant pressure), and the software will automatically read the data such as cell expansion thickness, expansion force, current, voltage, capacity, etc.
3. Result Analysis
As the number of cycles increases, the capacity decay of the cell and the thickness of the cell increases. As shown in Figure 3, the charging thickness expands by 3.4% at the beginning of the cycle, the discharge volume shrinks by 3.1%, and the irreversible expansion is about 0.3%. When the capacity retention rate is 80%, the maximum expansion thickness of the cell reaches about 20%, and the expansion curve also increases sharply as the capacity decays sharply.
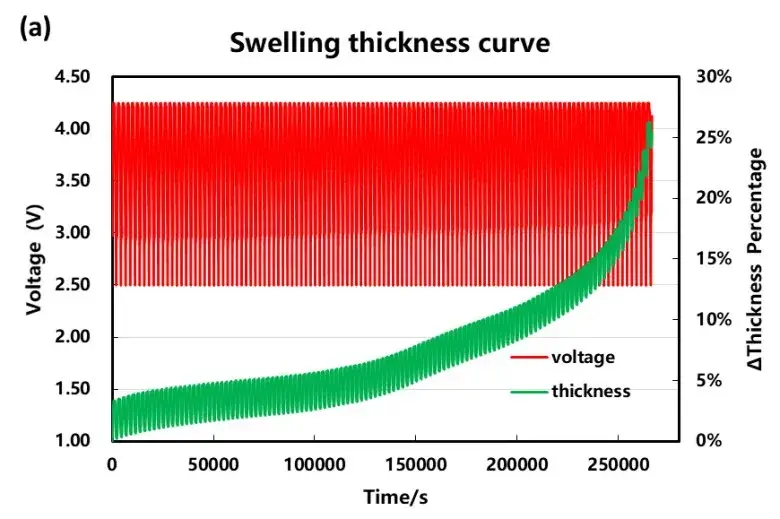
Figure 3. (a) Variation curve of charging and discharging voltage and swelling thickness
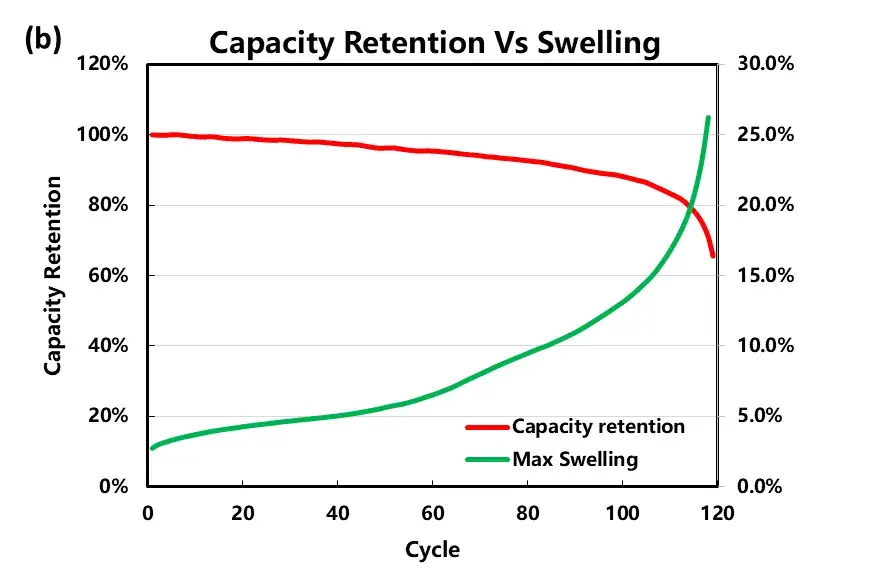
Figure 3. (b) Variation curve of charging capacity and charging maximum thickness
For NCM/graphite cells, lithium ions intercalate into graphite to gradually form Li-C compounds including LiC72, LiC36, LiC24, LiC12, and LiC6, which lead to the expansion of graphite lattice, and the microscopic stress generated by lattice expansion is the main driving force for electrode expansion. Electrodes are composed of active particles, binders, conductive additives, and pores formed between them. The lattice expansion caused by lithium intercalation is accompanied by the structural evolution of the binder and the change of the porous structure in the electrode. The pore structure evolution can change Li-ion transport and diffusion processes and associated stresses in electrode films during charge and discharge. The thickness evolution of the cell can be divided into the electrochemical expansion caused by the deintercalation process of lithium; the physical expansion caused by the volume evolution of polymers (such as binders and dispersants), electrode mechanical and structural changes. From the lattice expansion curve, the process of lithiation and delithiation is reversible. However, the continuous accumulation of stress in the microscopic lattice expansion of graphite may cause defects such as material structure damage and mechanical cracks in the electrode, while the mechanical or structural changes related to the electrode film are irreversible. Therefore, there may be several main reasons for the continuous increase of the irreversible thickness of the battery cell: the destruction of the electrode and material structure, side reactions, lithium precipitation, etc.
In order to further analyze the cause of the expansion, we select the charging thickness change curves corresponding to different cycle numbers, and analyze the difference after merging. The horizontal axis takes the charging capacity of the initial first cycle as 100%, and the vertical axis represents the thickness expansion of each cycle, as shown in the figure 4. As the number of cycles increases, it can be seen that the charging capacity of the battery cell continues to decrease, and after 110 cycles, the thickness expansion curve is obviously different from the previous expansion curve, especially at the later stage of charging, the slope of the expansion curve increases significantly, which can be It is speculated that after 110 cycles, the battery continues to accumulate stress during the charge-discharge cycle, irreversible mechanical damage has occurred, and side reactions such as lithium precipitation have occurred, which will cause the expansion rate of the battery cell to be greater than the initial expansion rate.
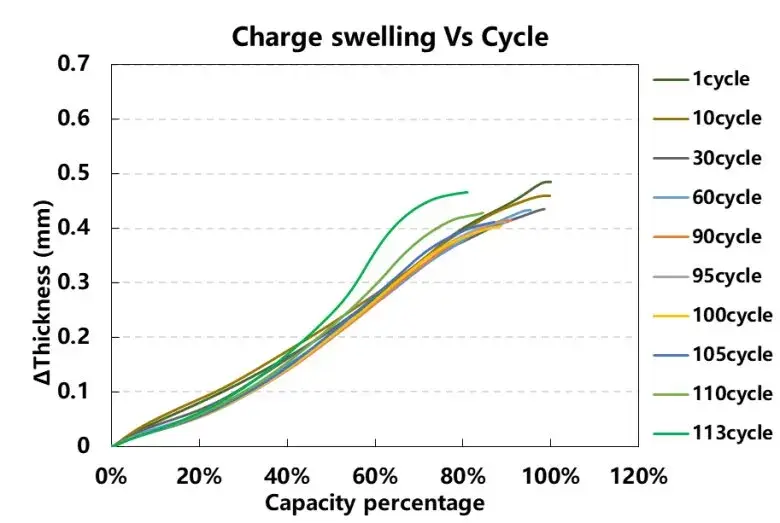
Figure 4. The change curve of the charging swelling force of each cycle of the battery cell
In addition, analyze the differential capacity curves corresponding to different cycle numbers, as shown in Figure 5. During the charging process, there are three characteristic peaks of charging phase transition transformation, and as the cycle increases, the voltage corresponding to each peak (as shown in Table 2) first decreases and then increases, that is, the cell polarization first decreases and then increases. It shows that applying a certain external pressure to the battery cell can reduce the polarization of the battery cell during the charging and discharging process at the beginning of the cycle, but with the continuous accumulation of subsequent side reactions, lithium precipitation, etc., it will increase the polarization of the battery cell. The intensity change of each peak is shown in Table 2 and Figure 6. Compared with the three characteristic peaks, the intensity change ratio is inconsistent, indicating that the cause of the cell cycle attenuation is not due to the structural damage of the active material, but mainly due to the mechanical damage of the electrode and lithium plating caused by side effects.
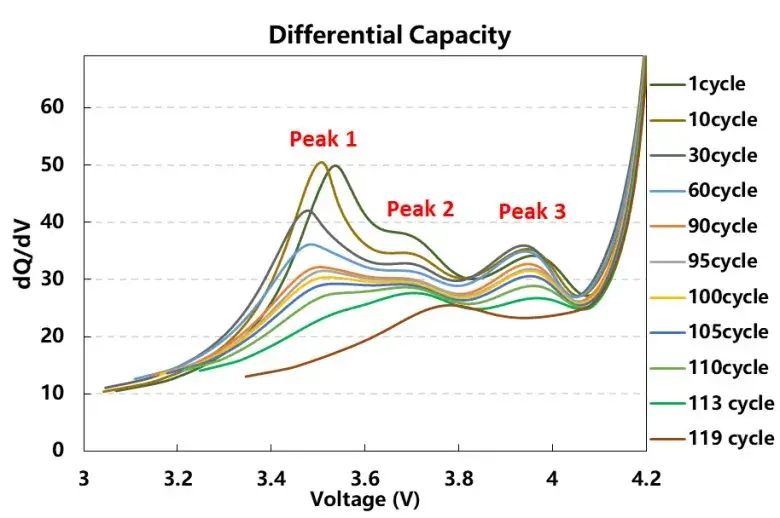
Figure 5. Differential voltage curve of cell charging swelling force
Table 2. Differential Voltages for Charging the Battery Cells
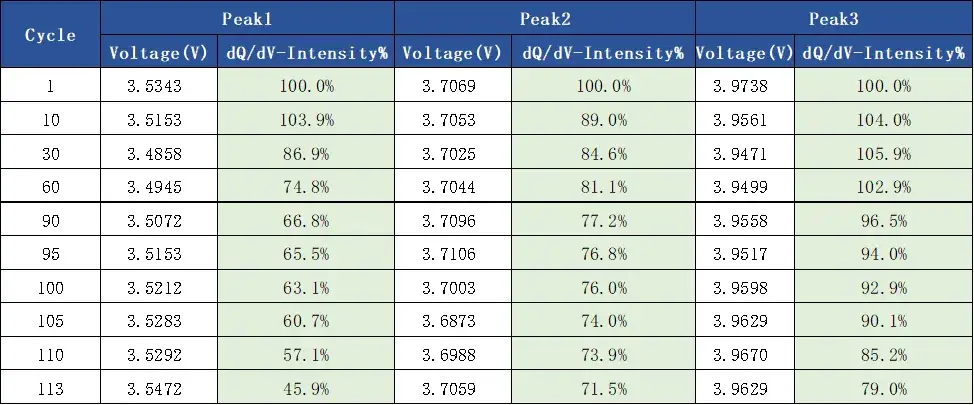
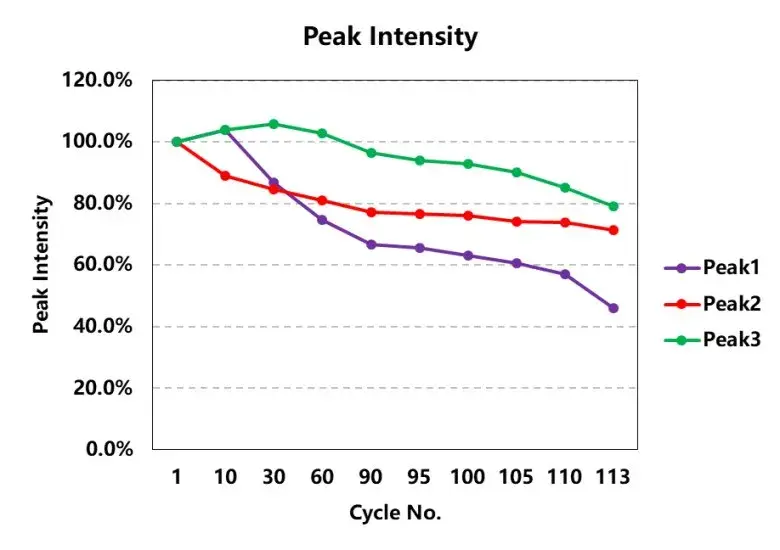
Figure 6. Trend chart of the peak intensity of each phase transition
4. Summary
In this paper, an in-situ swelling analyzer (SWE2110) was used to analyze the correlation between capacity decay and thickness swelling during the long cycling process of NCM cells, and through the analysis of the related cell swelling thickness and electrochemical data, it was hypothesized that the reasons for the cycling degradation of this cell included mechanical damage of the electrodes, lithium plating, and other side reactions.
5. References
[1] Huang K L, Lyu Z Z, Liu S Q. On capacity fading and its mechanism for lithium-ion batteries[J]. Battery Bimonthly, 2001,31(3): 142-145.
[2] Wang Q Y, Wang S, Zhang J N, et al. Overview of the failure analysis of lithiumion batteries[J]. Energy Storage Science and Technology, 2017, 6(5): 1008-1025.
[3] Christoph R. Birkl, Matthew R. Roberts, Euan McTurk, Peter G. Bruce, David A. Howey, Degradation diagnostics for lithium ion cells,Journal of Power Sources 341 (2017) 373-386.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



