-
iestinstrument
Expansion Decomposition and Comparison of Cathode and Anode Electrode for Lithium-ion Batteries
1. Preface
The cathode and anode electrodes of lithium-ion batteries undergo structural expansion or contraction due to de-embedded lithium during charging and discharging. When charging a lithium-ion battery, what happens on the anode electrode side is the process of lithium intercalation (such as graphite anode electrodes, hard carbon anode electrodes, etc.) or alloying lithium insertion (such as silicon-based anode electrodes, lithium metal anode electrodes, etc.) ,therefore, anode electrode materials generally undergo significant volume expansion as the depth of lithium embedding increases. For example, graphite anode electrodes generally produce a volume expansion of 10% to 15%, while silicon-based anode electrodes can produce a maximum volume expansion of 300%. However, for lithium battery cathode materials, what occurs during the charging process is a delithiation process, so will its structure shrink as the delithiation depth increases? The answer was “no”. Literature research shows that NCM or LCO cathode materials will also undergo structural expansion during charging and delithiation. This is because the removal of lithium ions will increase the interlayer Coulomb repulsion in the c-axis direction of the microcrystalline structure of the cathode material, resulting in macroscopic structural expansion[1,2].
Usually, people always use the whole battery as the main body to study the volume changes of the battery during the charge and discharge process. Although this method is simple to operate, the results can only reflect the overall lithium battery expansion of the cathode and anode electrodes, it is impossible to further decouple the expansion of the cathode and anode electrodes, and comparatively analyze the contribution proportions of the cathode and anode electrode materials to the overall expansion behavior of the full battery. Nor can it answer the above questions about the expansion behavior of the positive electrode materials.
So can we use a half-cell to decouple the expansion ratio of the cathode and anode electrodes? Since lithium sheets will undergo great volume expansion during the process of deintercalation and deintercalation of lithium [3], the traditional half-cell assembly method is still unable to effectively decompose the expansion behavior of the cathode and anode electrodes. Based on this, IEST adopts special structural design and processing technology to isolate the expansion interference of the lithium sheets in the half-cell, thereby effectively decoupling and analyzing the expansion of the cathode and anode electrode sheets!
2. Testing Conditions
2.1 Testing Equipment
The in-situ charge and discharge expansion test of the cathode and anode half-cells uses IEST‘s self-made monopole expansion test mold, while the expansion test of the button full battery uses IEST’s self-made model buckle mold. The structural diagrams of the two are respectively As shown in Figure 1(c) and (b). The changes in the expansion thickness of the two in different lithium insertion states were recorded in real time through the silicon-based anode electrode expansion in-situ rapid screening system (RSS1400, IEST) equipped with a high-precision thickness sensor, as shown in Figure 1(a).
Figure 1. (a) Silicon-based anode expansion in-situ rapid screen system (RSS1400, IEST); (b)The mold corresponding to the volume expansion of the full coin cell (b) half coin cell (c) The mold corresponding to the volume expansion of the half coin cell
2.2 In-situ Testing Process
①The positive electrode is made of NCM523 material, and the anode electrode is made of SiC material. First, it is assembled into a button full battery in IEST’s self-made model buckle (as shown in Figure 1(b)), and under the condition of 5kg preload force at a rate of 01C Charge and discharge, while recording the expansion curve of the button-type full battery in-situ.
②Then assemble the button half cells of NCM523 cathode electrode and SiC anode electrode respectively in the monopole expansion test mold (shown in Figure 1(c)), and charge and discharge at a rate of 01C under the condition of 5kg preload force. At the same time, the thickness expansion curve of the positive or anode electrode piece was recorded in-situ.
3. Results Analysis
Table 1 shows the charge and discharge capacity and efficiency of button half cells and button full cells after two cycles. The efficiency of cathode and anode half cells is slightly lower than that of commercial 2032 button cells, this is caused using a special fixture structure and a special ceramic diaphragm in the expansion mold of the monopolar piece. Since the charge and discharge capacity is positively related to the corresponding thickness expansion, and the capacity of the cathode and anode half cells is inconsistent with that of the full battery, if you want to compare the expansion behaviors of the three, you need to normalize their capacity, that is, the thickness expansion produced by the unit charge and discharge capacity of the three is compared.
Figure 2 shows the charge and discharge curves of three batteries in the second cycle of charging. We normalized them according to their respective capacity performance, the charge and discharge range of the full cell (NCM523 // SiC) and the positive half cell (NCM523 // Li) is 3~4.25V, while the charge and discharge range of the anode half cell (SiC // Li) is 0.005~2V. Figure 3 shows the expansion thickness changes of the three batteries during the second charge and discharge cycle. It can be seen from this that the thickness expansion of the full battery during charging and discharging mainly comes from the anode electrode side, and accounts for more than 80%, the volume expansion on the cathode side only accounts for less than 10%, which is consistent with test results in other literature [4,5]. In addition, according to relevant data, the volume expansion of the current mainstream cathode materials is roughly [4,5]: LFP-6.5%, LCO-1.9%, LMO-7.3%, NCM-6.5% (depending on Ni content), NCA-6%.
Table 1. Comparison of charge and discharge capacity and efficiency between cathode and anode electrode button half cells and button full cells after two cycles
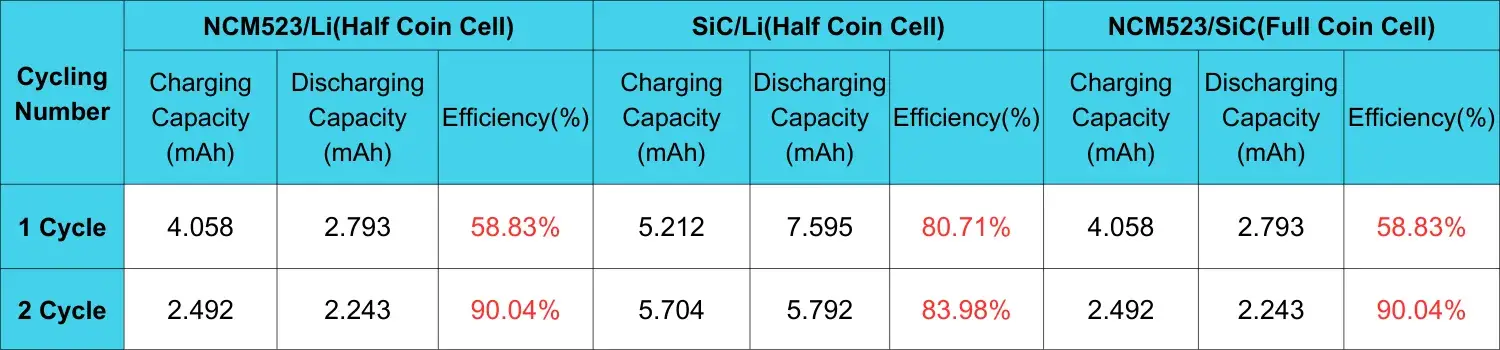
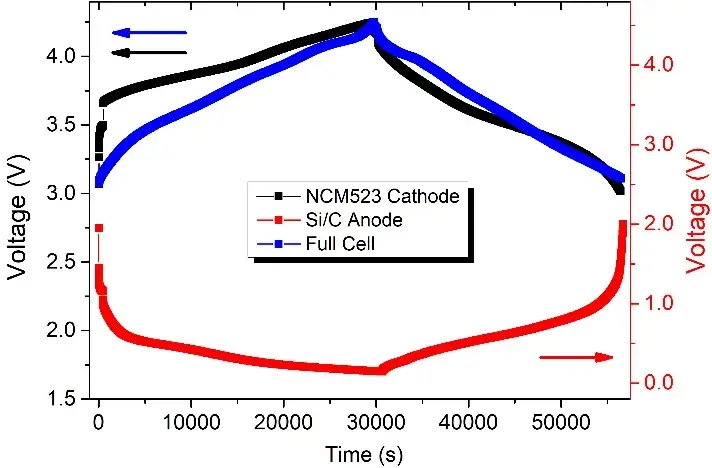
Figure 2. Voltage versus time curves for cathode and anode half coin cell and full coin cell for the second turn of charging and discharging. In order to facilitate the comparison of the three, the normalization was performed according to the capacity play.

Figure 3. Curves of unit capacity expansion versus time for the second turn of charge/discharge for cathode and anode half coin cell and full coin cell. In order to facilitate the comparison of the three, the normalization was performed according to the capacity play.
4. Summary
This paper uses the single-electrode expansion test mold developed by IEST to decompose and compare the expansion behavior of the cathode and anode electrodes of lithium-ion batteries. Because the mold uses a special structural design and a dedicated ceramic diaphragm, its charge and discharge efficiency is slightly lower than that of the commercial 2032 coin cell. However, it can still be seen from the expansion test results that the thickness expansion of the button-type full battery mainly comes from the negative electrode side, and accounts for more than 80%, while the volume expansion of the positive electrode side accounts for less than 10%, which is consistent with the test results in other literature[4,5]. This result helps researchers to compare and analyze the contribution of cathode and anode electrode materials to the volume expansion of the full battery, so as to optimize and modify materials more targetedly and accelerate the development of high-capacity, low-expansion materials!
5. References
[1] F.B. Spingler, S. Kucher, R. Phillips, E. Moyassari and A. Jossen, Electrochemically Stable In-Situ Dilatometry of NCM, NCA and Graphite Electrodes for Lithium-ion Cells Compared to XRD Measurements. J. Electrochem. Soc. 168 (2021) 040515.
[2] B. Rieger, S. Schlueter, S.V. Erhard and A. Jossen, Strain Propagation in Lithium-ion Batteries from the Crystal Structure to the Electrode Level. J. Electrochem. Soc. 163 (2016) A1595-A1606.
[3] C. Luo, H. Hu, T. Zhang, S.J. Wen, R. Wang, Y.N. An, S.S. Chi, J. Wang, C.Y. Wang, J. Chang, Z.J. Zheng and Y.H. Deng, Roll-to-Roll Fabrication of Zero-Volume-Expansion Lithium-Composite Anodes to Realize High-Energy-Density Flexible and Stable Lithium-Metal Batteries. Adv. Mater. 34 (2022) 2205677.
[4] R. Koerver, W.B. Zhang, L. Biasi, S. Schweidler, A.O. Kondrakov, S. Kolling, T. Brezesinski, P. Hartmann, W.G. Zeier and J. Janek, Chemo-mechanical expansion of lithium electrode materials – on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 11 (2018) 2142-2158.
[5] Y. Koyama, T.E. Chin, U. Rhyner, R.K. Holman, S.R. Hall and Y.M. Chiang, Harnessing the Actuation Potential of Solid-State Intercalation Compounds. Adv. Funct. Mater. 16 (2006) 492-498.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


