-
iestinstrument
Industry Challenges and Corresponding Solutions For Silicon-Based Anodes
With the vigorous development of the new energy industry, lithium-ion batteries are gradually developing in the direction of higher energy density and longer cycle life. The theoretical gram capacity of the existing graphite anode is only 372mAh/g, which can no longer meet the demand for battery energy density in the future. Silicon-based anodes have gradually become the next-generation lithium battery anode materials that can replace graphite due to their high theoretical gram capacity, rich content, and suitable lithium intercalation potential. However, silicon-based anodes also have pain points that limit their large-scale commercialization. This article summarizes some industry pain points in the production and use of silicon-based anodes materials, as well as the corresponding solutions that IEST can provide.

1. Large Volume Expansion
The lithium storage mechanism of the silicon anode is alloyed lithium storage. Unlike Lithium intercalation of graphite, silicon particles will cause huge volume expansion and contraction during the alloying/dealloying process. When silicon and lithium form a Li15Si4 phase, the corresponding maximum volume expansion can reach 300% [1]; due to the addition of oxygen atoms, the expansion rate of the silicon-oxygen anode can be reduced to 120%, but it is still far greater than the 10% to 12% of the graphite anode. The huge volume expansion will lead to the pulverization of silicon material particles, which will make the electrical contact between silicon particles and conductive agent worse; secondly, it will lead to continuous rupture and regeneration of the SEI film. This process will consume a large amount of active lithium and electrolyte, thereby accelerating the capacity decay and aging of the battery.
Nano-coating with carbon coated is one of the effective methods to solve the expansion of silicon anode. Studies have shown that as long as the silicon particles are reduced to less than 150nm, the expansion rate will drop significantly from 300% to about 30%, and then the outer layer will be coated with carbon, which can act as a buffer layer, thereby further reducing the volume expansion of the silicon-carbon anode Generally, silicon-carbon materials can be prepared by methods such as chemical vapor deposition, high-energy ball milling, and pulsed laser deposition, the main coating structures are divided into the following four types: (1) directly wrapping the carbon shell on the nano-silicon particles, similar to glutinous rice balls; (2) leaving a layer of space while wrapping the nano-silicon particles, like eggs; (3) Use two pieces of carbon materials to clamp nano-silicon particles to make a structure similar to a hamburger; (4) Similar to a watermelon, in which the watermelon seeds are nano-silicon particles, the watermelon flesh is loose graphite, and the watermelon rind is carbon deposition.
The porous design is also one of the means to effectively reduce the volume expansion of the silicon-carbon anode, which reserves pores for the volume expansion of the silicon-carbon anode material, so that the entire particle or electrode does not produce obvious structural changes. The methods for making voids generally include: (1) preparation of hollow Si/C core-shell structure materials; (2) preparation of Yolk-Shell structure Si/C composite materials; (3) preparation of silicon sponge structures, etc. In order to facilitate R&D personnel to quickly compare and evaluate the expansion of silicon-based materials with special structural designs, IEST has also launched a silicon-based anodes expansion in-situ fast screening system (RSS1400, IEST). The equipment uses the model buckle to conduct the in-situ expansion test at the pole piece level, which is not only easy to operate, but also greatly saves the test cost, and shortens the expansion evaluation cycle of silicon-based materials from dozens of days to 1-2 days. The physical picture of RSS1400 is shown in Figure 1(a), and the expansion comparison results of silicon-carbon materials with different structural designs are shown in Figure 1(b).
Figure 1. (a) Silicon-based anodes expansion in-situ rapid screening system (RSS1400, IEST); (b) Comparison of expansion of silicon-carbon materials with three different structural designs.
The use of appropriate binders can also limit the expansion of silicon particles and effectively inhibit particle pulverization, thereby improve the cycle stability of silicon-based materials. Traditional PVDF only relies on weak van der Waals force to connect with silicon-based anodes materials, and cannot adapt to the drastic volume change of silicon particles [2]. At present, the binders of silicon-based anodes materials that have been studied more are water-based binders such as CMC and PAA. Among them, SBR/CMC has good viscoelasticity and dispersibility, and has been widely used in the large-scale production of graphite anode, the molecular structure of PAA is simple and easy to synthesize, and S. Komaba et al. [3] also found that: PAA can form a coating layer similar to SEI film on the surface of silicon particles, thereby effectively inhibiting the decomposition of the electrolyte, so it is more suitable for silicon-based materials than CMC. IEST also used the self-developed in-situ expansion analysis system (SWE2110, IEST) to conduct in-situ expansion analysis on silicon-carbon batteries made of four different binders, and it can effectively quantitatively evaluate the expansion inhibition effect of the four binders, and the results are shown in Figure 2. In addition, other binders such as sodium alginate, carboxymethyl chitosan, and polyacrylonitrile can also be used in silicon-based anodes materials, and their expansion inhibition effects can also be tested and quantitatively evaluated by using SWE2110.
Figure 2. In-situ expansion analysis system (SWE2110, IEST) and the comparison of the expansion thickness of silicon-carbon anodes under the action of four different binders
2. The Problem of Gas Production in Homogenate
Although methods such as surface modification or element doping can effectively reduce the expansion of silicon-based anodes these improvement processes are often accompanied with unstable factors, for example, surface alkalinity and incomplete coating will cause nano-silicon to be exposed and react with hydroxide ions to generate gas during pulping. In addition, pre-magnesium or pre-lithiation treatment of silicon oxide can improve the first effect of silicon oxide, but at the same time, it also brings processing problems to the homogenate coating process of silicon oxide, such as gas production from homogenate, coating dropout, etc.2
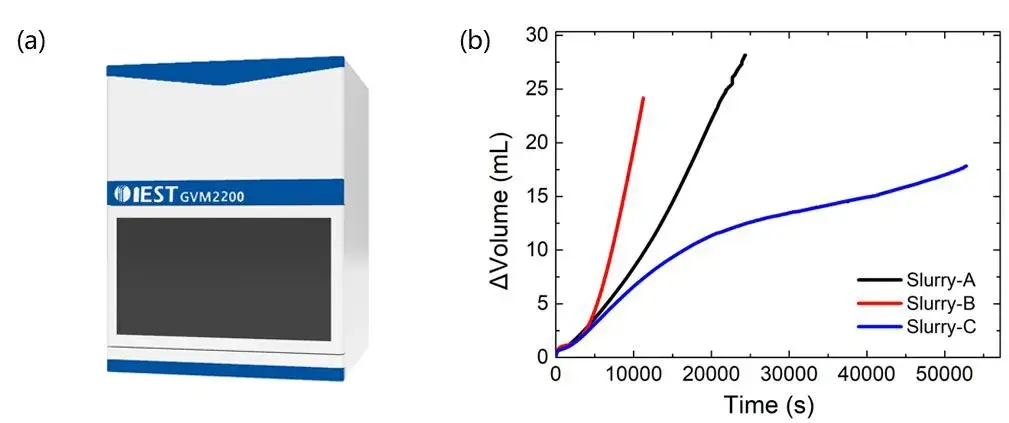
The IEST in-situ gas production volume monitor (GVM2200, IEST) can monitor the gas production behavior of the silicon-based anodes slurry in real time and quantitatively (as shown in Figure 3(a)). It is equipped with high-precision sensors that can effectively monitor small changes in gas production (with a resolution of up to 1μL), assisting R&D personnel to reveal the mechanism of slurry gas production and formulate effective suppression measures. Figure 3(b) shows the variation of gas production of three different SiC slurries with homogenization time when homogenized in aqueous solvent. From the point of view of slope, slurry B produced gas most rapidly; and from the point of view of gas production, slurry A produced most gas. This result can assist researchers to adjust the modification process of silicon-based materials and accelerate the development of high-performance silicon-based anodes materials.
Figure 3. (a) In-situ gas production volume monitor (GVM2200, IEST); (b) the gas production of three different SiC slurries with the homogenization time when homogenized in an aqueous solvent.
3. Poor Electrical Conductivity
The conductivity of the material particles plays an important role in the performance of the battery, especially the rate performance of the battery. When the conductive agent is unevenly dispersed or the electrical contact with the[Y1] active particles is poor, electrons cannot be effectively transported in the electrode, resulting in large polarization and a between battery aging. Silicon particles are nearly 100 million times less conductive than carbon materials, and SiOx is even less electronically conductive than silicon.
Carbon encapsulation and adding appropriate conductive agents can significantly improve the electronic conductivity of silicon-based materials. Commonly used carbon sources include phenolic resin, glucose, graphene oxide, carbon nanotubes, etc., among which carbon nanotubes are one of the most important conductive materials in silicon-based materials, in particular, single-walled carbon nanotubes, whose good flexibility and strong van der Waals force are one of the core factors to ensure the cycle stability of silicon-based anodes materials. At he same time, carbon nanotubes can also act as a buffer for the expansion of silicon particles, thereby further improving the cycle life of silicon-based anodes. In evaluating the electrical conductivity of silicon materials, IEST powder resistance meter (PRCD3100, IEST) can be used for testing. The device (as shown in Figure 4) has two different resistance testing functions integrated of two probes and four probes at the same time, and can evaluate and compare the conductivity of different carbon-coated silicon-based materials. In addition, the equipment can also carry out a variable pressure test of up to 200MPa to provide users with the change of resistance and compaction density of silicon-based powder under different pressures, so as to guide the rolling process of silicon-based anodes.
Figure 4. The physical picture and test principle of the powder resistance meter (PRCD3100, IEST) and the comparative evaluation of the electrical conductivity of different carbon-coated silicon-based anode materials.
4. Low First Effect
The battery needs to be formed before use, and an SEI film is formed on the surface of the silicon particles. Since silicon particles are generally nano-sized and have a large specific surface, the film-forming process consumes a large amount of active lithium from the electrolyte or positive electrode material, as a result, the capacity during charging cannot be fully utilized during the first discharge, that is, the first effect is low (irreversible capacity loss can be as high as 10% to 30%). Compared with silicon-carbon materials, the first effect of silicon-oxygen materials is worse, which is also one of the important factors that limit the commercialization of silicon-oxygen materials.
The pre-lithiation process can effectively improve the first effect of silicon-based materials, especially silicon-oxygen materials. Its technical route includes two types of saying itheium supplementation at the negative electrode and lithium supplementation at the positive electrode.Among them, the negative electrode lithium supplement technology has received more attention and research because of its high lithium supplement capacity and clear technical route. At present, the main processes include lithium foil lithium supplementation, lithium powder lithium supplementation and other lithium supplementation methods, among which: (1) lithium foil lithium supplementation is a technology that uses the self-discharge mechanism to supplement lithium. The lithium sheet can be directly pressed on the negative electrode surface, and the potential difference between the lithium sheet and the electrode sheet can be used to insert lithium ions into the negative electrode. Although this method is simple to operate, it is difficult to control the degree of pre-lithiation, and it is easy to cause insufficient or excessive lithium supplementation. (2) Lithium supplementation with lithium powder was firsly roposed by FMC Corporation, and the surface of the stabilized lithium metal powder (SLMP) developed by them as coated with a thin layer of lithium carbonate of 2% to 5%. It an be directly sprayed onto the surface of the dry negative electrode for lithium supplementation, or added during the process of slurry mixing.
Although the lithium supplementation of the negative electrode has a high lithium supplementation capacity, the operation is complicated and has high environmental requirements. In contrast, the positive electrode lithium supplement material can be directly added in the homogenization process, which has good compatibility with the existing battery production process, and is safe, stable and low in price, so it is known as the most promising lithium supplement technology. Generally speaking, positive electrode lithium supplements can be mainly divided into the following three categories: one is to use binary lithium-containing compounds to supplement lithium, such as Li2O,Li2O2 and Li3N. This type of substance has a high specific capacity, and only a small amount of addition can achieve the lithium supplement effect, but the disadvantage is that it has poor stability, and it is easy to decompose and generate gas during the actual homogenization and lithium supplement process. Theas production can also be monitored in real time by using the IEST in-situ gas production volume monitor (GVM2200, IEST). The specific experimental process is shown in Figure 5. The second is to use lithium-rich compounds to replenish lithium, such as Li5FeO4 and Li2NiO2; the third is to use lithium compounds to replenish lithium, such as Li2S/Co,LiF/Co and Li2O/Co. These types of substances have their own advantages and disadvantages. Therefore, in the future, positive electrode lithium supplement materials need to be developed in the direction of high chemical stability, low decomposition potential, no gas production, and high lithium delithiation capacity.
![]()
Figure 5. Flowchart of measuring Si content in silicon carbon materials using in-situ gas volume monitor (GVM2200, IEST)
5. Monitoring of Material Composition Proportion
Quickly measuring the silicon-carbon ratio, silicon-oxygen ratio or nano-silicon content in silicon-based anodes materials, not only can ieffectively also quickly estimate the specific capacity of the material, which is of great significance for companies to improve research and development efficiency. The carbon content, oxygen content and silicon content in silicon-based anodes materials can be tested separately by high-frequency infrared carbon-sulfur analyzer, oxygen-nitrogen-hydrogen analyzer, silicon-molybdenum blue spectrophotometry and X-ray diffraction.
The high-frequency infrared carbon-sulfur meter can effectively calibrate the carbon content in the silicon-based anodes material. During the test, 0.05g of the silicon-based material and 1.5-1.8g of tungsten-tin particle flux can be weighed in the ceramic crucible, and fully mixed evenly , then it can be burned and tested with oxygen in a high-frequency infrared carbon-sulfur meter; The oxygen, nitrogen and hydrogen analyzer can be used to determine the oxygen content in the silicon-oxygen anode. During the test, about 0.03g of the silicon-oxygen anode material can be weighed and melted in an inert gas-protected pulse electrode furnace, and the oxygen content can be tested by infrared absorption method ; Silicon-molybdenum blue spectrophotometry can also be used to detect the silicon content in materials. This method mainly uses strong alkali to melt SiO2 in the material at high temperature, ammonium molybdate is added after adjusting to a suitable pH value, and the concentration of SiO2 is detected by spectrophotometry. Since both Si and SiO2 react with strong bases, it is also not possible to effectively distinguish Si and SiO2. At the same time, high-temperature alkali leaching has higher requirements on the material of the container (high temperature resistance, acid and alkali resistance, etc.), and deviations are prone to occur during operation.
X-ray phase analysis has the characteristics of non-destructive, rapid and reproducible. As a semi-quantitative test method, it has three advantages: one is non-destructive test, there is no chemical reaction, which eliminates the error caused by reaction by-products; second, the operation is simple, the test cycle is short, and the test efficiency is high; the third is that it uses less material and has a better discrimination between different substances.
6. Summary
Silicon-based andoes materials have become the most commercially promising next-generation anode materials, and their technical routes are mainly divided into silicon carbon and silicon oxygen. The more mature commercial products at this stage are mainly silicon-oxygen materials, but in the future they will gradually move closer to silicon-carbon materials. This article summarizes some industry pain points in the production and use of silicon-based anodes material, such as volume expansion, homogenate gas production, poor conductivity, and low first effect. In order to solve these problems, it is not only necessary for upstream materials companies and downstream cell companies to integrate advantageous resources to accelerate the development of micro-mechanisms and preparation processes, but also for testing equipment companies to develop convenient, fast and effective testing instruments. As a comprehensive solution provider in the lithium battery testing industry, IEST is committed to providing the most professional solutions for the research and development of silicon-based anodes materials in terms of expansion, gas production, and electrical performance testing, and to help the large-scale commercialization of silicon-based anodes materials!
7. References
[1] M. Ashuri, Q.R. He and L.L. Shaw, Silicon as a potential anode material for Li-ion batteries: where size, geometry and structure matter. Nanoscale 8 (2016) 74–103.
[2] Z.H. Chen, L. Christensen, and J.R. Dahn, Large-volume-change electrodes for Li-ion batteries of amorphous alloy particles held by elastomeric tethers. Electrochem. Commun. 5 (2003) 919-923.
[3] S. Komaba, K. Shimomura, N. Yabuuchi, T. Ozeki, H. Yui and K. Konno, Study on polymer binders for high-capacity SiO negative electrode of Li-ion batteries. J. Phys. Chem. C 115 (2011) 13487-13495.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.