-
iestinstrument
Application of EIS Test To Lithium Battery On Pressure Condition
1. Abstract
This study uses electrochemical impedance spectroscopy (EIS testing) to evaluate how external module pressure and state-of-charge (SOC) influence the impedance of LCO/graphite prismatic cells. Combining an in-situ swelling analyzer (SWE2110) with a Princeton PARSTAT MC workstation, we performed stepwise compression and EIS diagnostic tests (10,000 Hz → 0.02 Hz, 5 mV) at pressures from 100–1000 kg (≈0.27–2.7 MPa). Key findings: the EIS test low-frequency real component (and charge transfer resistance, Rct) increases with pressure—this effect is strongest at low SOC and much weaker at high SOC. The imaginary part (double-layer capacitance) is essentially unchanged by pressure.
2. Introduction — why EIS diagnostic testing matters
EIS diagnostic tests are non-destructive and reveal internal electrochemical dynamics (ohmic resistance, SEI, charge transfer, diffusion). For battery pack designers and cell manufacturers, understanding how compression from module packaging influences EIS spectra is critical for safety and performance: packaging preload can change contact resistance, electrode porosity, ionic pathways and even trigger particle fracture. This work links EIS testing with practical module-level concerns (preload and recommended SOC at assembly).
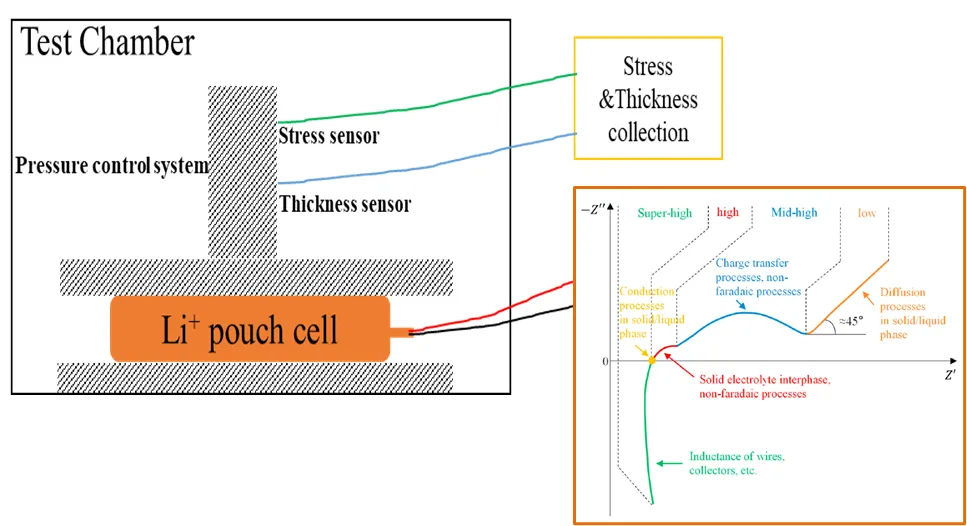
2. Experimental Setup and Method
2.1 Equipment
-
In-situ swelling analyzer (SWE2110, IEST): Allows precise application and monitoring of mechanical pressure on pouch cells.
-
Princeton PARSTAT MC electrochemical workstation: Performs high-precision EIS test measurements across a frequency range of 10 kHz to 0.02 Hz.
Figure 1. (a) Appearance of SWE2110 equipment; (b) Princeton Electrochemical Workstation
2.2 Test Information and Process
2.2.1 The cell information is shown in Table 1
Table 1. Information of Test Cell
2.2.2 Test Process
-
Five LCO/graphite pouch cells were prepared at SOC levels of 0%, 20%, 40%, 60%, and 80%.
-
Each cell was subjected to stepwise pressures from 100 kg to 1000 kg (0.27–2.7 MPa) using the SWE2110.
-
After 20 minutes of pressure stabilization, EIS testing was conducted to record Nyquist and Bode plots.
3. Result Analysis
3.1 Nyquist Diagram Analysis of EIS Test at Different Pressures
The EIS diagnostic test revealed two semicircles in the impedance spectra: a high-frequency segment associated with SEI film resistance, and a mid-to-low frequency region corresponding to charge transfer processes. Under increasing pressure, the low-frequency region (≤ 0.125 Hz) shifted significantly toward higher impedance, especially in low-SOC cells (e.g., 0% SOC). This indicates increased charge transfer and diffusion resistance under mechanical load. High-SOC cells (e.g., 80% SOC) showed minimal shift, suggesting better pressure tolerance.
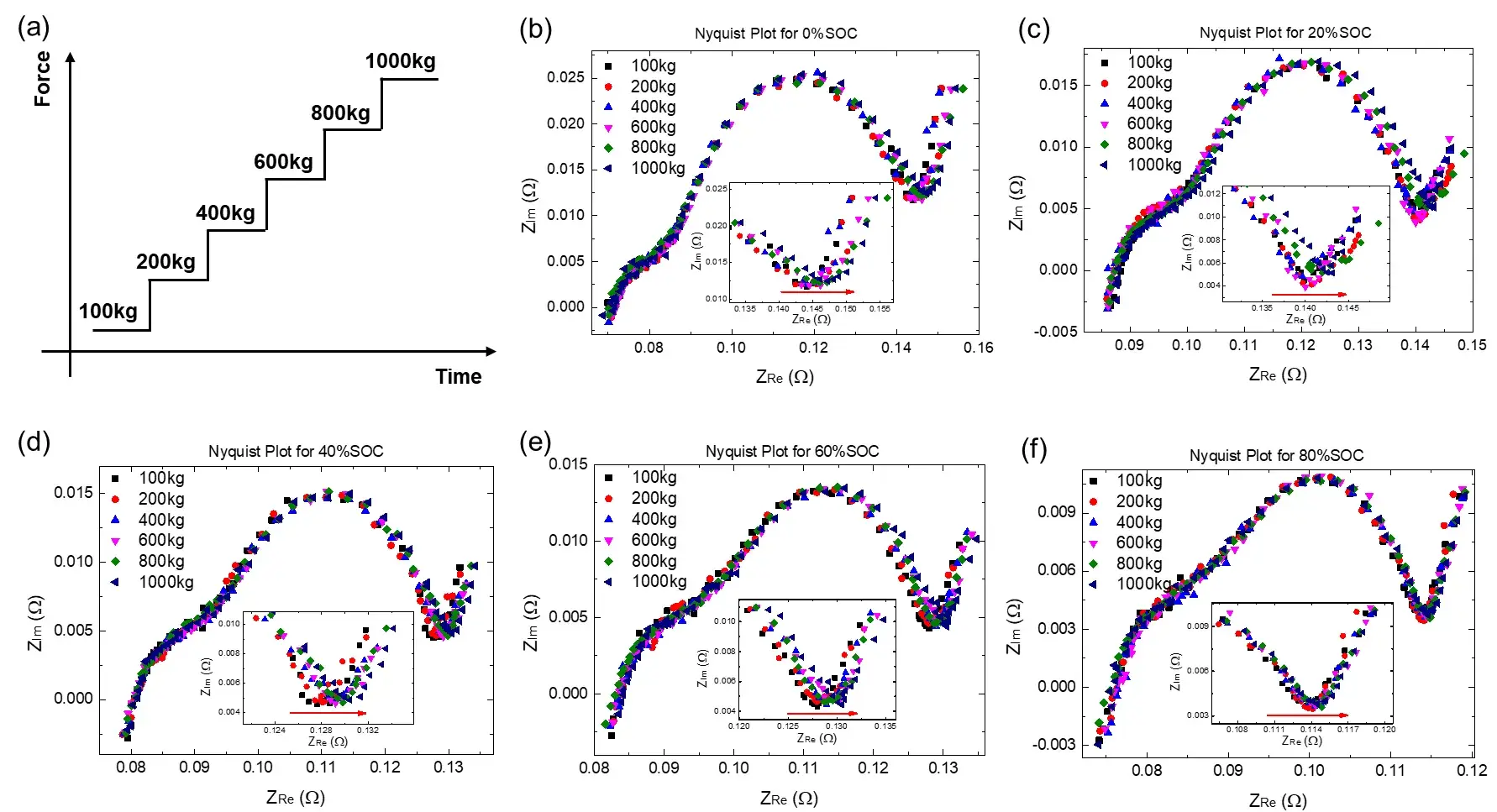
Figure 2. (a) Schematic diagram of stepped pressurization; (b-f) The EIS spectra of cells with 0%, 20%, 40%, 60% and 80% SOC respectively under different pressures (100kg, 200kg, 400kg, 600kg and 1000kg)
3.2 Bode Plot Analysis
Further, we selected three different SOCs of high (80%), medium (40%), and low (0%), and analyzed the Bode plots of these three cells under different pressures, as shown in Figure 3. It can be seen that the imaginary part of the EIS test of these three SOC cells has no significant change under all pressures, no matter in the high-frequency region or the low-frequency region (as shown in Figure 3(b), (d), (f) ), However, different pressures mainly have obvious effects on the real part of the EIS in the low frequency region (as shown in Fig. 3(a), (c), (e)). In addition, it can also be seen from the insets of Figure 3(a), (c), and (e) that as the SOC increases, the increasing trend of the real part of the low-frequency region becomes less obvious, which is consistent with the previous results analyzed from the Nyquist diagram, indicating that the higher the SOC, the less susceptible the real part of the low-frequency EIS to the pressure.
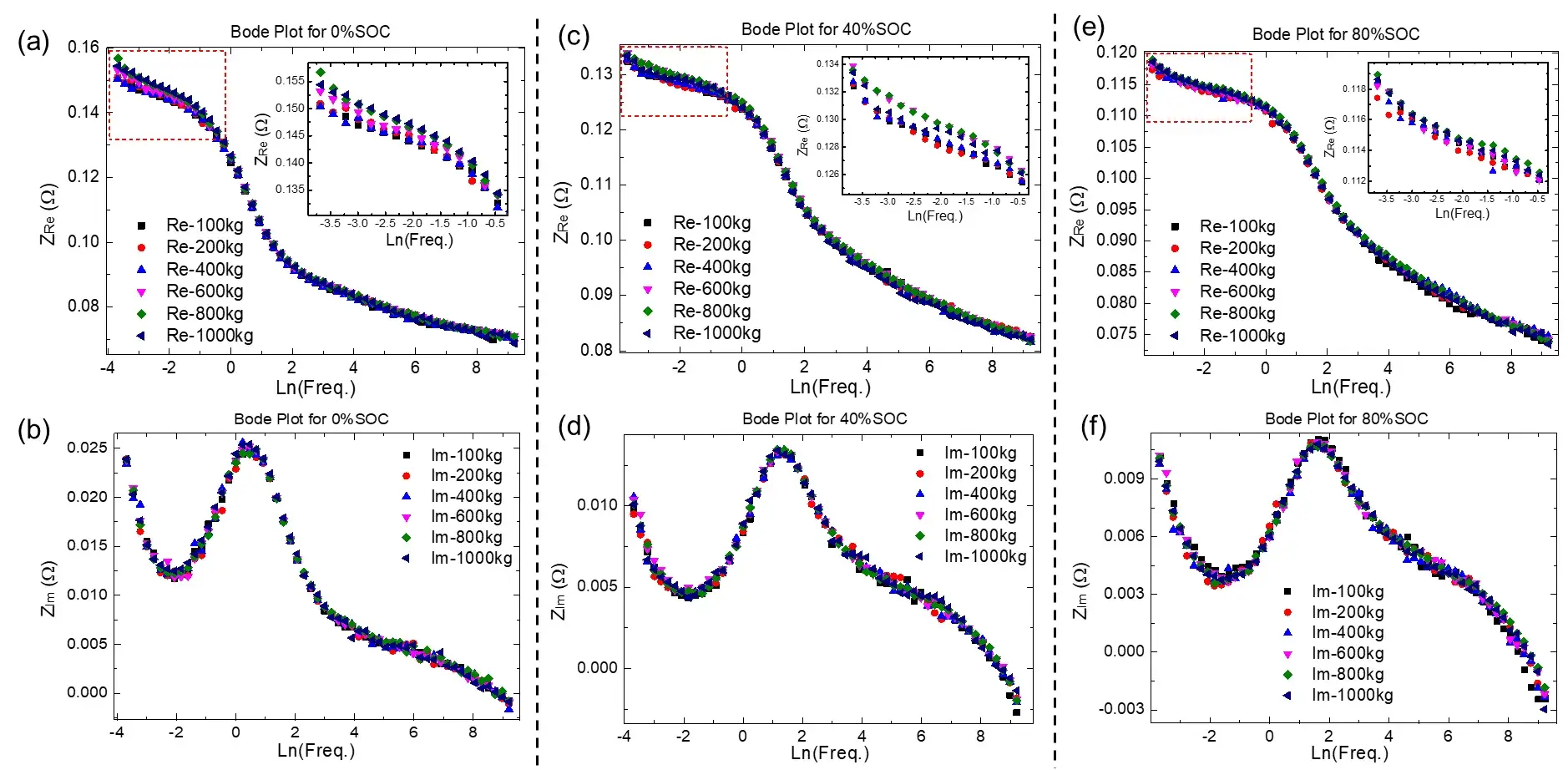
Figure 3. (a-b) are the real and imaginary parts of the 0% SOC cell as a function of frequency; (c-d) are the real and imaginary parts of the 40% SOC cell as a function of frequency; (e-f) are the real part and imaginary part of the 80% SOC cell as function of frequency.
3.3 Equivalent Circuit Analysis
Using a simplified Randles model (Figure 4), Rₑₜ was quantified for each SOC under varying pressures. At 0% SOC, Rₑₜ increased by ~3.78 mΩ under 1000 kg load, whereas at 80% SOC, the increase was only ~1.34 mΩ. This divergence is attributed to structural changes in the graphite anode: at low SOC, graphite layers compress readily, increasing van der Waals forces and impeding Li⁺ diffusion. At high SOC, intercalated lithium provides structural support, reducing compressibility and resistance increase.
However, it can be seen from the previous discussion that the real part of the EIS in low-frequency region increases continuously with the increase of pressure, and this phenomenon is more significant at low SOC. In order to quantitatively study-this phenomenon, we extracted the Rct of different SOC cells under different pressures, and the results are shown in Figure 4(c). It can be seen that when the pressure increases from 100kg to 1000kg, the Rct of the 0% SOC cell increases ~3.78 mΩ, however, the Rct of the 80% SOC cell only increases~1.34 mΩ, and the higher the SOC, smaller increase of the Rct with increasing the pressure. On the one hand, the external pressure will cause the positive and negative coatings to be compressed and deformed, the porosity of the active coating will decrease, the ion transport resistance will increase, and even the particles will be compressed and broken, which will eventually lead to an increase in Rct. On the other hand, when the cell is at 0% SOC, there is almost no intercalation of lithium between the layers of the graphite negative electrode, so it is easier to be compressed. When a certain pressure is applied, the interlayer spacing of the graphite layer is gradually reduced during the compression process, and the van der Waals force between the layers increases¹. At this time, the charge transfer process of Li⁺ and the subsequent diffusion and intercalation process will be greatly affected. making the diffusion impedance in the low frequency region increase significantly. When the cell is at 80% SOC, the graphite negative electrode is close to the state of fully intercalated lithium. At this time, the graphite layer can withstand greater pressure without being significantly compressed. Therefore, although the 1000kg of pressure is also applied, to the cell with 80% SOC, the increase of the van der Waals force of the graphite negative electrode is not as obvious as that of the cell with low SOC cell. Thus, the charge transfer of Li⁺ and its subsequent diffusion and embedding process are less resisted than those of low-SOC cells, so the Rct of cell with 80% SOC only increases about ~1.34 mΩ under high pressure, which is only 35% of the increasement of that at 0% SOC. Therefore, when it is necessary to apply a certain preload to the cell (for example, when packaging a module), we can predict that if the initial SOC of the cell is high, the preload will not have a significant impact on the cycling performance of the cell. However, when the initial SOC of the cell is relatively lower, the excessive preload may reduce the lithium-interactable capacity of the graphite negative electrode, and affect the cycle efficiency of the cell.
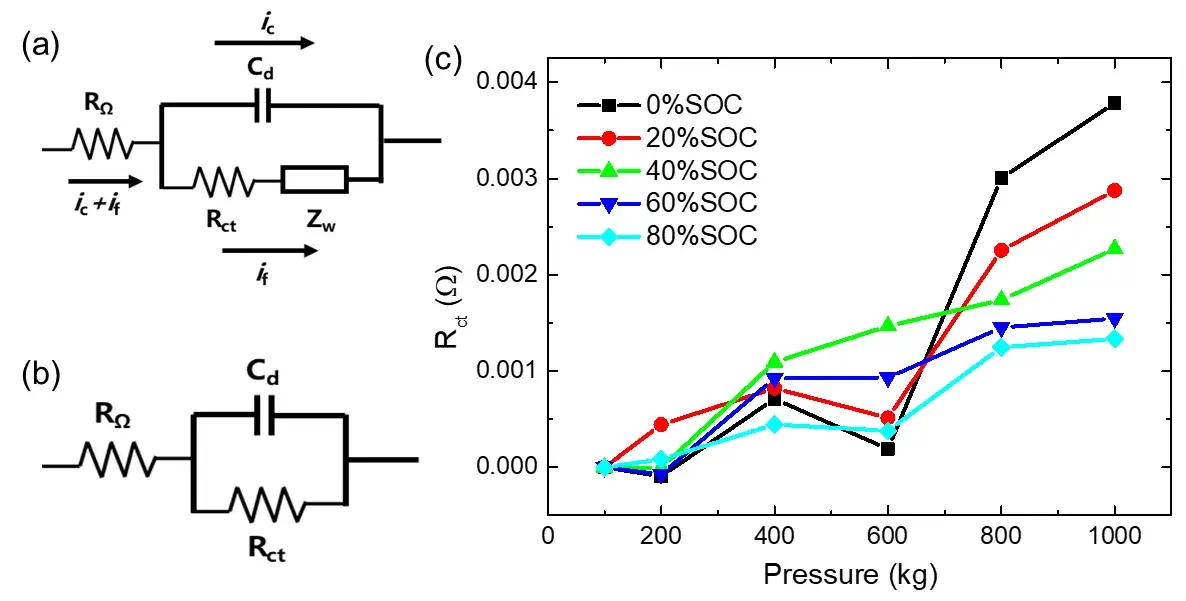
Figure 4. (a) is the Randles equivalent circuit commonly used in lithium-ion batteries, and the shunt situation when the current passes through the working interface; (b) is the simplified equivalent circuit after ignoring the Warburg impedance when the frequency is high; (c) is the change of charge transfer resistance Rct of different SOC cells with different pressures
4. Mechanistic discussion (from EIS diagnostic data)
-
Why low-frequency increases? Pressure reduces electrode porosity and tortuosity, restricting ion pathways and increasing diffusion impedance (Warburg contribution). Particle crushing or coating deformation at high pressure can further raise Rct.
-
Why SOC matters? At high SOC the graphite negative electrode is already lithiated and mechanically more resistant to compression; low SOC graphite (delithiated) compresses more, amplifying the impedance rise.
-
Practical implication: Preload applied during module assembly will have less negative impact when cells are assembled at higher SOCs.
5. Practical recommendations (based on EIS testing results)
- Prefer higher SOC during module pre-loading: If the assembly process requires significant pre-tightening, charge cells to a higher SOC to reduce Rct increases post-assembly.
- Limit excessive structural preload: Avoid unnecessary over-compression that may permanently reduce electrode porosity and increase diffusion resistance.
- Use EIS diagnostic tests as QC: Run baseline EIS testing after assembly to detect excessive Rct or diffusion impedance increases linked to mechanical stress.
- Design for even pressure distribution: Non-uniform preload can create local high-impedance zones and accelerate degradation.
- Monitor low-frequency EIS features: Changes at low frequency are the earliest spectral marker for pressure-induced transport limitations.
4. Summary
This study demonstrates that EIS diagnostic test methods are sensitive to mechanical pressure and SOC-dependent electrochemical behavior. Key findings include:
-
Low-frequency impedance increases significantly under pressure, especially in low-SOC cells.
-
The double-layer capacitance remains stable across all pressure conditions.
-
Applying pressure at high SOC mitigates increases in charge transfer resistance.
These insights underscore the importance of pressure management during battery packaging and support the use of EIS test protocols in battery manufacturing and R&D for enhanced performance and safety.
5. References
[1] H.M. Lu, H.F. Fang, X.M. He and L.Q. Xie, Effect of pressure on charge and discharge performance and expansion of ternary lithium battery. J. Power Technol. 41 (2017) 686-688.
[2] W.X. Hu, Y.F. Peng, Y.M. Wei, Y. Yang, Application of Electrochemical Impedance Spectroscopy to Degradation and Aging Research of Lithium-Ion Batteries. J. Phys. Chem. C 127 (2023) 4465-4495.
[3] Q.C. Zhuang, Z. Yang, L. Zhang and Y.H. Cui, Research process on diagnosis of electrochemical impedance spectroscopy in lithium-ion batteries. Prog. Chem. 32 (2020) 761-791.
[4] Allen J Budd, Larry R Faulkner, Electrochemical Methods-Principles and Applications [M], Second Edition, Chemical Industry Press, 2005.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.