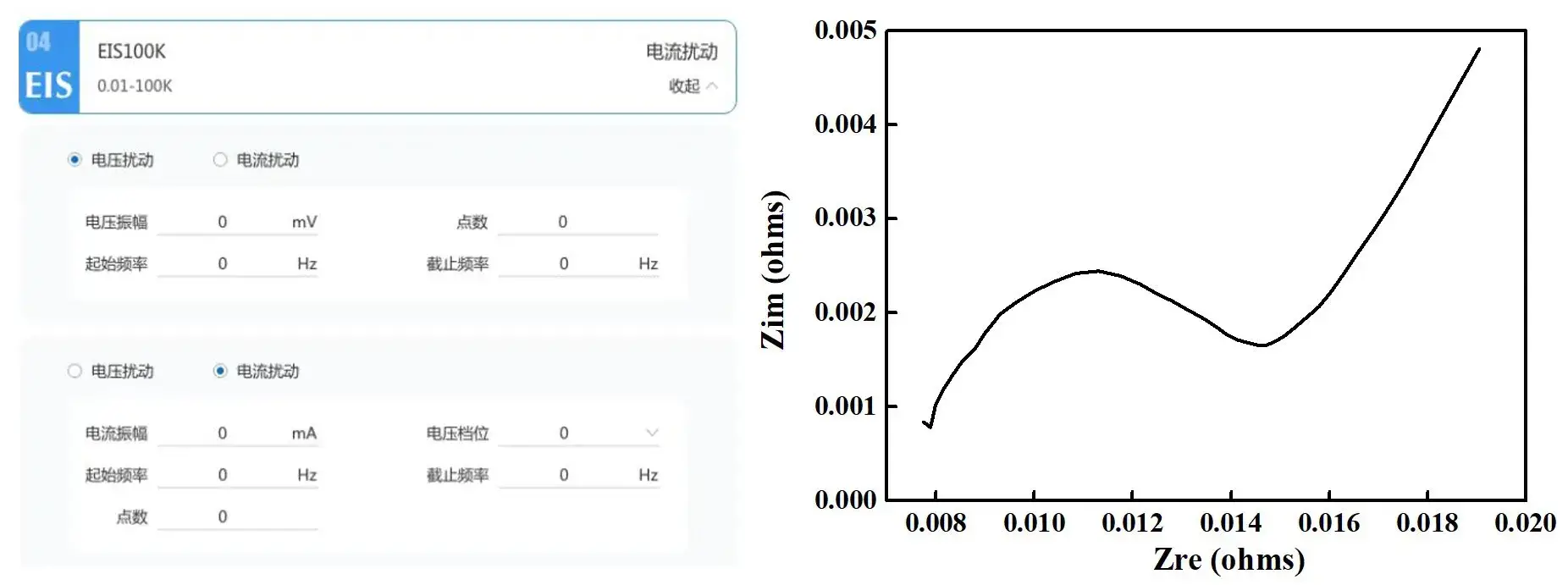
-
iestinstrument
Entering Electrochemistry | The Secret Weapon of Electrochemical Research—The Three Electrode System
Have you heard of the three electrode system? It might be completely different from the batteries we use in our daily lives, which typically have two electrodes: a positive and a negative one. So, why is there a three electrode system? What does its structure look like? Why can’t we see the third electrode in a battery? What is its purpose? Today, let’s explore the wonders of the three electrode system.
1. What is the Three Electrode System?
The three electrode system consists of three electrodes: the Working Electrode (WE), the Counter Electrode (CE), and the Reference Electrode (RE). Each electrode has its unique function and role.
1.1 Working Electrode (WE)
This is the core of the research. In electrochemical experiments, chemical reactions primarily occur on the surface of the working electrode. It is like a test field in the laboratory where we observe and record the process and results of reactions. The working electrode must meet several conditions: ① The studied electrochemical reaction should not be influenced by reactions occurring on the electrode itself, and it should accommodate a wide voltage range. ② The electrode must not react with the solvent or electrolyte components. ③ The electrode area should not be too large, its surface should be uniform and smooth, and it should be capable of being easily cleaned. The working electrode is usually “inert.” Common solid electrodes include glassy carbon, platinum, gold, silver, lead, and conductive glass (FTO, ITO, etc.). Common liquid electrodes include liquid mercury. When using solid electrodes, to ensure reproducibility of the experiment, it is important to establish appropriate electrode pretreatment procedures.
1.2 Counter Electrode (CE)
Also known as the auxiliary electrode, it forms a current loop with the working electrode, allowing current to flow in the electrochemical cell. The main function of the counter electrode is to balance the current of the working electrode, ensuring the stability of the current in the entire system. The counter electrode is typically made of highly conductive and chemically stable materials, such as platinum or graphite.
1.3 Reference Electrode (RE)
This is a potential-stable electrode that does not participate in the reaction, providing a constant reference potential. The importance of the reference electrode lies in its ability to provide an accurate reference point for the potential of the working electrode, ensuring the accuracy of electrochemical measurements. Common reference electrodes include the Saturated Calomel Electrode (SCE) and the Silver/Silver Chloride Electrode (Ag/AgCl).
2. Why is the Three Electrode System So Important?
In the early days of electrochemical research, experiments were primarily conducted using a two-electrode system. Although simple, the two-electrode system had significant drawbacks, particularly in measuring and controlling electrode potentials, leading to considerable errors. In the 1920s, electrochemists began to introduce the reference electrode, thereby inventing the three electrode system. This innovation greatly improved the precision and reproducibility of electrochemical experiments. Simply put, the introduction of the three electrode system brought two key benefits to electrochemical research:
2.1 Precise Potential Control
The introduction of the reference electrode allows us to independently measure and control the potential of the working electrode without being influenced by the current. This independence greatly enhances the precision of experiments, especially when studying the kinetics and mechanisms of electrochemical reactions, providing more reliable data.
2.2 Analysis of Complex Electrochemical Systems
In a two-electrode system, measurement results are often affected by various factors such as the electrolyte, electrode materials, and current paths, leading to poor accuracy and reproducibility of data. In the three electrode system, the reference electrode provides a stable potential reference, allowing us to more clearly separate and analyze the different components and processes within the electrochemical system.
2.3 The Structure and Connection of the Three Electrode System
The three electrode system typically needs to be used in conjunction with an electrochemical workstation or charge-discharge equipment to provide voltage and current to the system while accurately measuring voltage and current. It can be simply understood as connecting an ammeter between the working electrode and the counter electrode, and a voltmeter between the working electrode and the reference electrode. The three electrode system consists of two circuits: one for measuring electrode potential and the other for measuring current, which is referred to as the “three-electrode, two-circuit” setup.
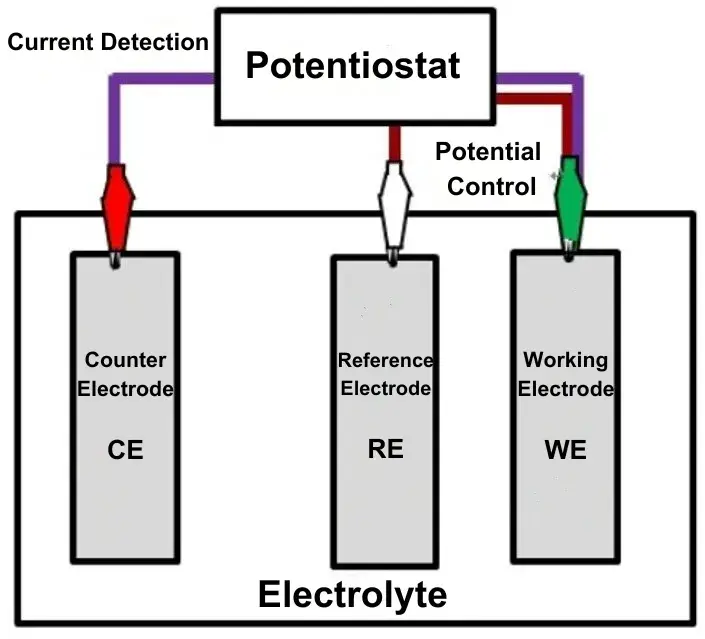
Figure 1. Structure of the Three Electrode System
In electrochemical research, particularly in studies related to batteries, the use of the three electrode system has become a standard method. This approach offers precise potential control and independent current measurement. However, to fully leverage the advantages of the three electrode system, high-precision electrochemical analyzers are required to complement its usage. Below are several key reasons along with detailed explanations.
Figure 2. Example with the IEST Electrochemical Analyzer
3. Precise Potential Control
The reference electrode in the three electrode system provides a stable potential reference for precise control and measurement of the working electrode potential. High-precision electrochemical workstations feature high sensitivity and low noise characteristics, enabling the maintenance of potential stability even at extremely low currents. This is crucial for studying minute electrochemical changes in batteries. For instance, when investigating the negative electrode materials of lithium-ion batteries, the potential range for the formation of SEI films is typically narrow. Electrochemical analyzers such as the IEST can accurately control and measure potential changes at the microvolt (μV) level, thereby obtaining reliable data.
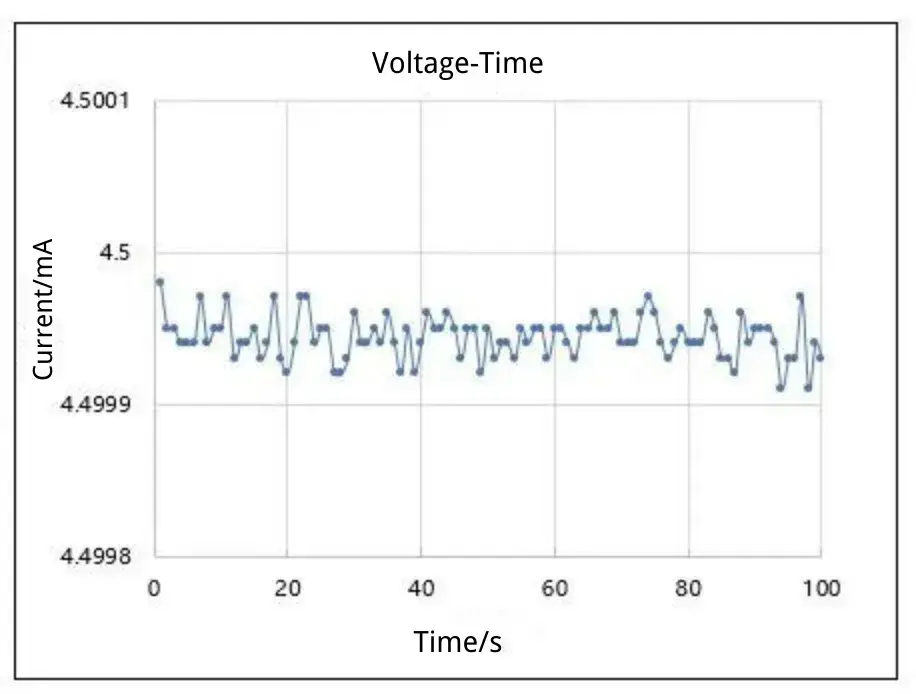
Figure 3. Voltage Control Example: Setpoint: 4.5 V Measured Value: 4.5 V ± 100 μV
4. Accurate Current Measurement
The dynamics and mechanisms of electrochemical reactions require precise measurement of current responses. The IEST electrochemical analyzer can provide current measurement accuracy up to the nanoampere level (nA), which is crucial for analyzing parameters such as charge transfer impedance and reaction rates of battery materials. For instance, in cyclic voltammetry (CV) testing, researchers need to measure peak currents and potentials of electrode reactions. The IEST electrochemical analyzer enhances industry-standard current measurement accuracy to 0.01%, providing accurate current data to ensure the precision and repeatability of CV curves.
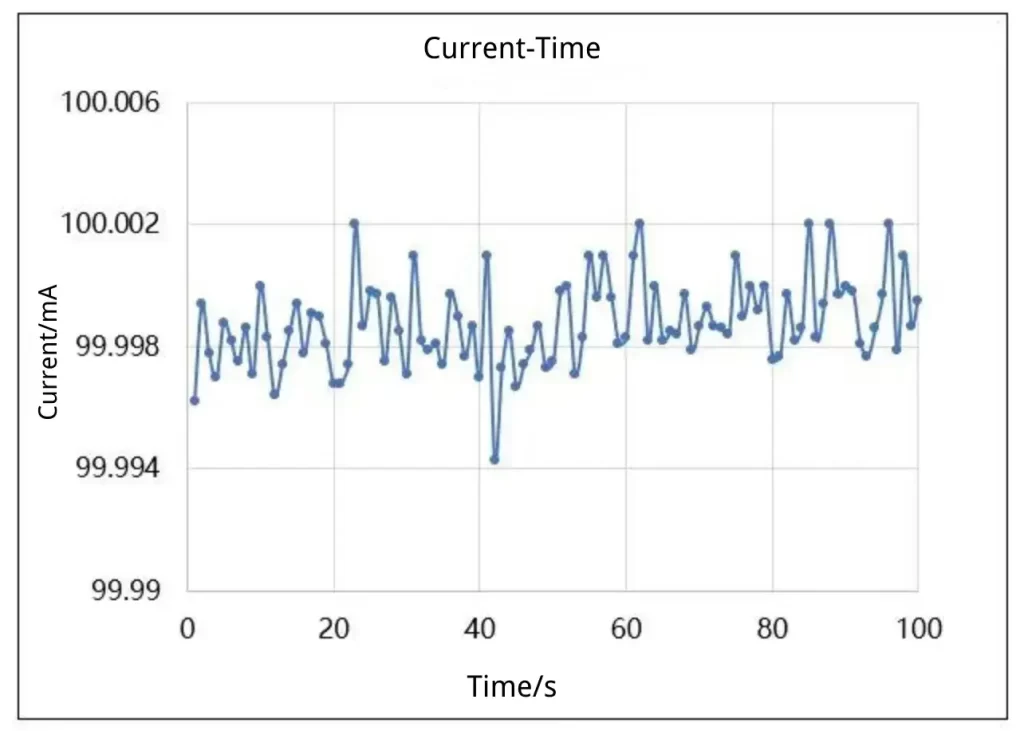
Figure 4. Current Control Example: Setpoint: 100 Ma Measured Value: 100 mA ± 5 μA
5. Low Noise Characteristics
Electrochemical signals are often very weak and susceptible to interference from external environmental noise. The IEST electrochemical analyzer is designed with various anti-interference techniques to effectively reduce noise and enhance signal purity. This is crucial for obtaining high-quality experimental data. For example, in electrochemical impedance spectroscopy (EIS) testing, high-frequency noise may affect the impedance measurement results in the high-frequency region. The IEST R&D team has developed an integrated solution for EIS modules with low-noise design, allowing for more accurate EIS spectra, aiding researchers in better analyzing the internal resistance and interface characteristics of batteries.
Figure 5. Setting up EIS Steps and Displaying EIS Data on the IEST Electrochemical Analyzer
6. Wide Frequency Response Range
Electrochemical impedance spectroscopy (EIS) testing involves a broad frequency range from low to high frequencies. The IEST electrochemical analyzer features a wide frequency response range, covering frequencies from 0.01 Hz to 100 kHz, catering to diverse research needs. For instance, studying the ion diffusion processes in batteries typically requires low-frequency EIS data, while investigating charge transfer reactions in electrode materials necessitates high-frequency EIS data. The high-precision IEST electrochemical analyzer can provide consistent and accurate measurement results across the entire frequency range.
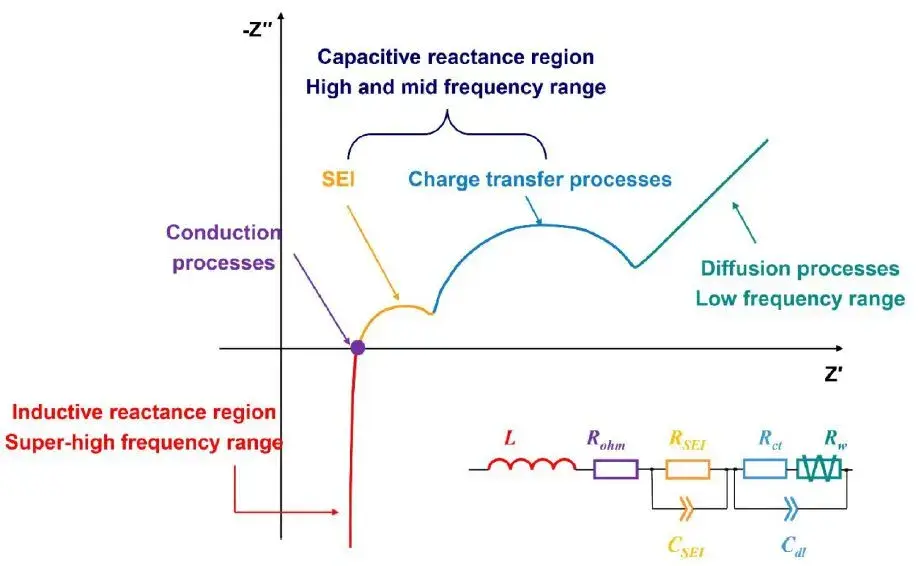
Figure 6. Physical and Chemical Properties of Electrochemical Systems Characterized by EIS in Different Frequency Ranges
7. Multi-functional Integration
The high-precision IEST electrochemical analyzer integrates various electrochemical testing techniques such as cyclic voltammetry (CV), potentiostatic intermittent titration technique (PITT), galvanostatic intermittent titration technique (GITT), and others. The combined use of these techniques allows for comprehensive characterization of the thermodynamic and kinetic properties of battery materials. In addition to the conventional charge-discharge functions, the IEST electrochemical analyzer integrates commonly used CV and EIS modules found in electrochemical workstations, catering to diverse applications across various scenarios for customers.
8. Data Processing and Analysis
The IEST electrochemical analyzer is equipped with advanced data processing and analysis software capable of real-time processing and multidimensional analysis of complex electrochemical data. This capability is highly beneficial for understanding the behavior of battery materials and optimizing battery performance. While hardware parameters are not the primary limiting factors for the localization of current electrochemical workstations, optimizing circuits and devices can indeed improve the results of electrochemical testing to some extent. However, the increase in costs and the associated “benefits” have not been fully recognized by the market. There is an urgent need for intelligent data analysis software that not only showcases data but also allows for refined data analysis. Of course, the IEST electrochemical analyzer is also evolving in this direction.
9. Summary
Through this article, readers have gained a deeper understanding of the three electrode system. From a user perspective, although we cannot see the three-electrode structure in the actual use process of battery cells, it plays a crucial role in the early laboratory stages of battery development. Through EIS measurements, impedance characteristics of the SEI film can be resolved to understand its impact on battery performance; through CV measurements, the redox reaction mechanism and kinetic parameters of electrode materials can be analyzed; and through PITT and GITT measurements, the chemical potential and diffusion coefficients of electrode materials can be obtained to optimize material design and battery performance. Overall, the introduction of the three electrode system provides feasibility for precise research of complex electrochemical systems. We look forward to the continued unique advantages of the three electrode system contributing to the development of electrochemical science.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.