-
iestinstrument
A Guide to the Dry Electrode Process: Assessment Methods for Next-Generation Battery Manufacturing
1. Abstract
Dry electrode technology is emerging as a key enabler for next-generation battery formats — including large-format cylindrical cells, semi-solid/solid-state cells, and high-capacity designs — because it eliminates solvent use, reduces capital and energy costs, and enables thicker, crack-free electrodes. This article presents a practical metrology workflow for evaluating dry electrode powder and film quality using powder resistivity & compaction density measurements (PRCD3100, IEST) and electrode resistance tester (BER2500, IEST). We summarize experimental procedures, key test parameters, and diagnostic readouts that link PTFE fibrillation, feed/processing conditions, and roll-press pressure to powder/electrode resistivity and compactness. The methods help R&D engineers compare materials and processing windows rapidly and quantitatively, accelerating optimization of dry electrode process parameters and formulations.
2. Background
Lithium-ion batteries dominate the field of new energy vehicles and energy storage due to their high energy density, high power, and long cycle life. As commercialization advances, demands for lower manufacturing costs and higher performance intensify. The cost and performance of Li-ion batteries are largely determined by their electrode manufacturing process. Therefore, innovative, reliable, and low-cost electrode fabrication technologies are crucial for widespread battery adoption.
Currently, most advanced commercial lithium-ion batteries utilize slurry casting for electrode manufacturing. Figure 1 shows a schematic of this process. It involves mixing active anode or cathode powders, conductive additives, and binders into a solvent to form a stable suspension. Typically, deionized water is used for anodes, while the organic solvent N-Methyl-2-pyrrolidone (NMP) is used for cathode slurry preparation. The slurry is then cast onto a current collector using a slot-die coater and dried in an oven tens of meters long. Drying, conducted at high temperatures often exceeding 100°C to evaporate the solvent quickly, consumes significant energy. Furthermore, due to the potential environmental hazards of NMP, cathode manufacturing requires expensive and complex NMP recovery systems, further increasing costs.

Figure 1. Schematic diagram of the electrode manufacturing process using slurry coating technique
The push for higher energy density also hinges on manufacturing thick electrodes, a area where slurry casting faces severe limitations. Commercial electrodes are typically less than 100 μm thick. Using slurry casting for thicker electrodes often causes binder migration during drying. When the binder floats to the electrode surface, it leads to low binder content within the electrode bulk, compromising cohesion between particles and adhesion at the electrode/current collector interface. This reduced mechanical integrity can lower production yield and cause capacity fade.
In contrast, the dry electrode process, which eliminates solvents, can potentially overcome all limitations associated with thick electrode manufacturing. In this process, the binder, active material, and conductive additives are homogenized dry, avoiding uneven binder distribution and enabling the production of thick electrodes. Using thicker electrodes significantly boosts energy density. Consequently, the dry electrode process is gaining considerable attention as one of the most promising solutions for reducing manufacturing costs and improving electrode quality.
3. Advantages of the Dry Electrode Process
-
Cost Reduction: By eliminating solvents and associated evaporation, recovery, and drying equipment, the dry electrode process can significantly lower production costs. One study suggests potential savings of up to 56% for producing one million lithium-ion cells.
-
Elimination of Delamination: This technology enables uniform distribution of electrode components without solvents, thereby avoiding electrode delamination caused by solvent evaporation.
-
Enablement of High-Areal-Capacity Electrodes: The process is well-suited for fabricating thick electrodes, allowing for better control over thickness and uniformity. This makes it ideal for producing ultra-high-loading electrodes.
-
Environmental Friendliness: The solvent-free nature of the technology effectively reduces environmental pollution.
Over the past five years, Tesla has announced the adoption of the dry electrode process for producing the next generation of batteries. Volkswagen also claims to have made significant progress in dry electrode technology. The increasing number of patents filed over the past decade indicates a rapid growth in industrial research on dry electrode manufacturing, particularly in dry powder spray deposition and polymer fibrillation methods. The roll-to-roll dry electrode process is currently a primary focus of research. Figure 2 shows the Maxwell dry electrode process flowchart.
Despite the many advantages of the dry electrode process, it also faces some challenges, such as the difficulty in controlling the uniformity and consistency of electrode film formation. These challenges encompass several aspects, including process ratio optimization, adjustment of mixing process parameters, and anomaly detection in the process. Among these, detection is the most challenging part. Ensuring uniformity during the dry electrode film formation process is of utmost importance. This article, based on the detection indicators related to the dry electrode process, outlines IEST current exploration and understanding in the evaluation and detection segment of the dry electrode process.
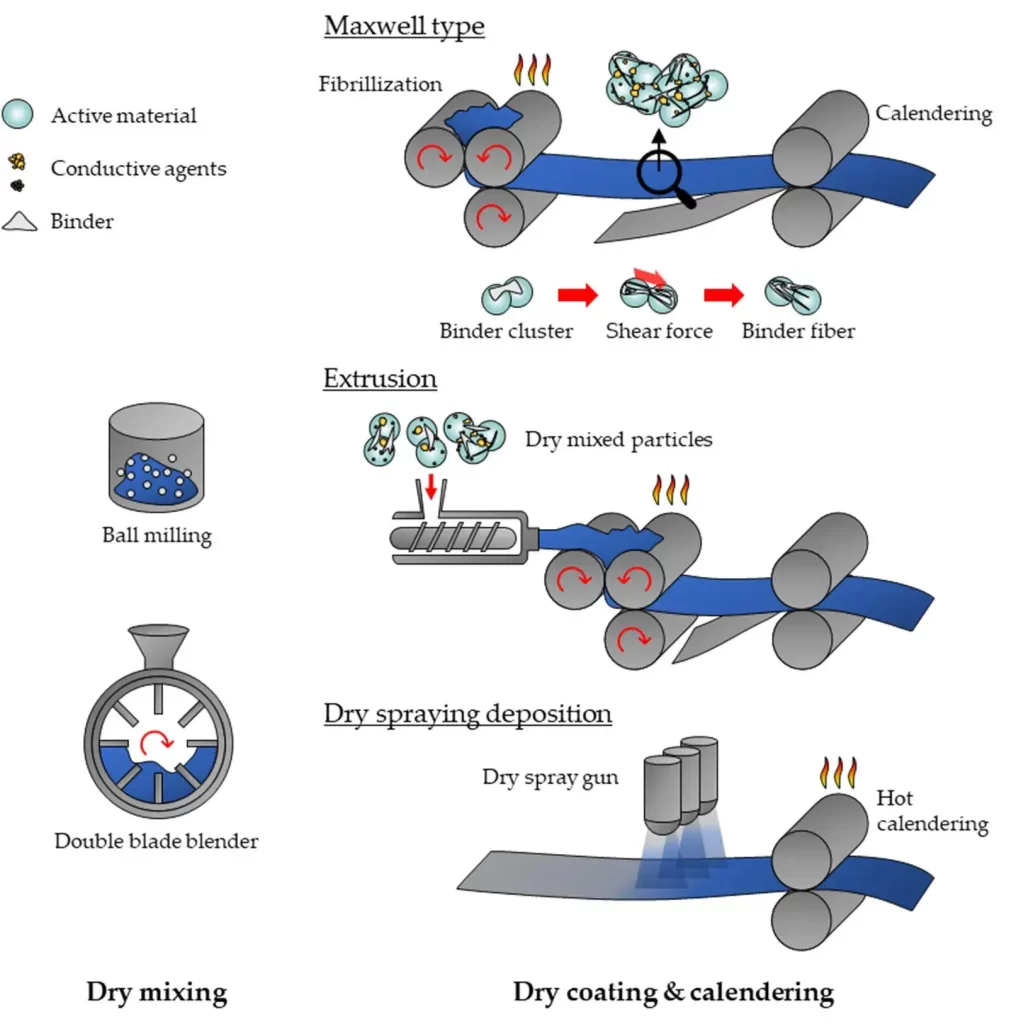
Figure 2. Maxwell dry electrode process flowchart
4. Evaluation of Dry Electrode Manufacturing Process
The rolling-based dry electrode manufacturing typically involves three steps: dry mixing, dry coating (dry deposition), and final calendering to achieve the desired thickness and dense electrode structure. The uniformity of the dry mixing step critically impacts subsequent stages. The formulation used in dry mixing ultimately affects the electronic and ionic transport paths, the final electrode’s compaction density, and even cell-level electrochemical performance.
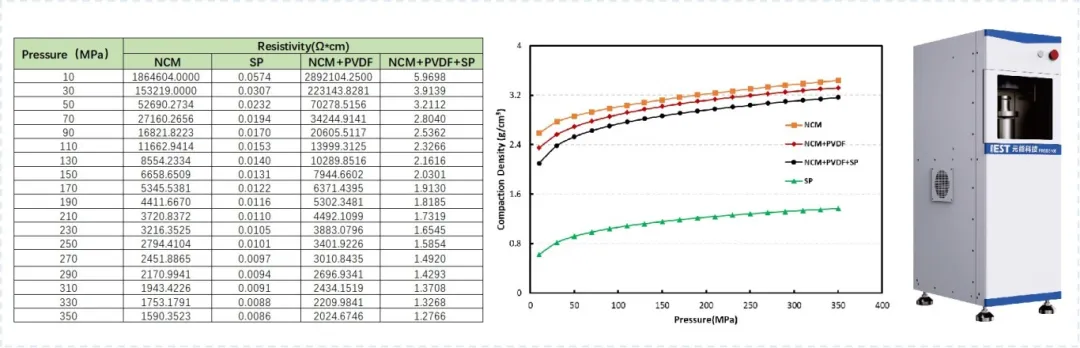
Powder resistivity and compaction density are critical metrics for lithium battery R&D and process evaluation. Figure 3 shows results from measurements using the PRCD series instruments on “dry-mixed” powders. In this experiment, cathode materials, binders, and conductive agents were mixed in different ratios (NCM:PVDF=19:1 and NCM:PVDF:SP=18:1:1) to simulate the mixing stage of the dry electrode process. The results clearly show distinct conductivity and compaction density behaviors with the introduction of conductive agents and binders.
In a typical dry process, the core material in the dry mixing stage is a fibrillatable binder like Polytetrafluoroethylene (PTFE). This is mixed with active material and conductive agents. During mixing, ensuring uniform dispersion of all components is essential. Simultaneously, under shear forces, the binder is physically stretched from its original spherical form into fine fibrils (a process called “fibrillation”). This creates a fibrous network that connects the active material particles, providing excellent binding. The material ratios and mixing parameters are crucial for achieving uniform dispersion. Monitoring resistivity and compaction density during the mixing stage provides valuable theoretical support for optimizing formulations and process parameters.
Figure 3. Results of powder resistivity and compaction density determination for “Dry Mixing”
Recent tests on dry electrode samples in our laboratory indicate a direct correlation between the resistivity of the mixed powder and the resistivity of the calendered electrode sheet. This finding further validates the effectiveness of assessing powder conductivity at the mixing stage.
While powder characterization is key early on, evaluation at the dry coating and final calendering stages focuses on the electrode sheet level, which is critical for predicting final electrochemical performance. Our laboratory systematically assesses the electronic conductivity and ionic transport properties of dry electrodes using resistance and tortuosity measurements.
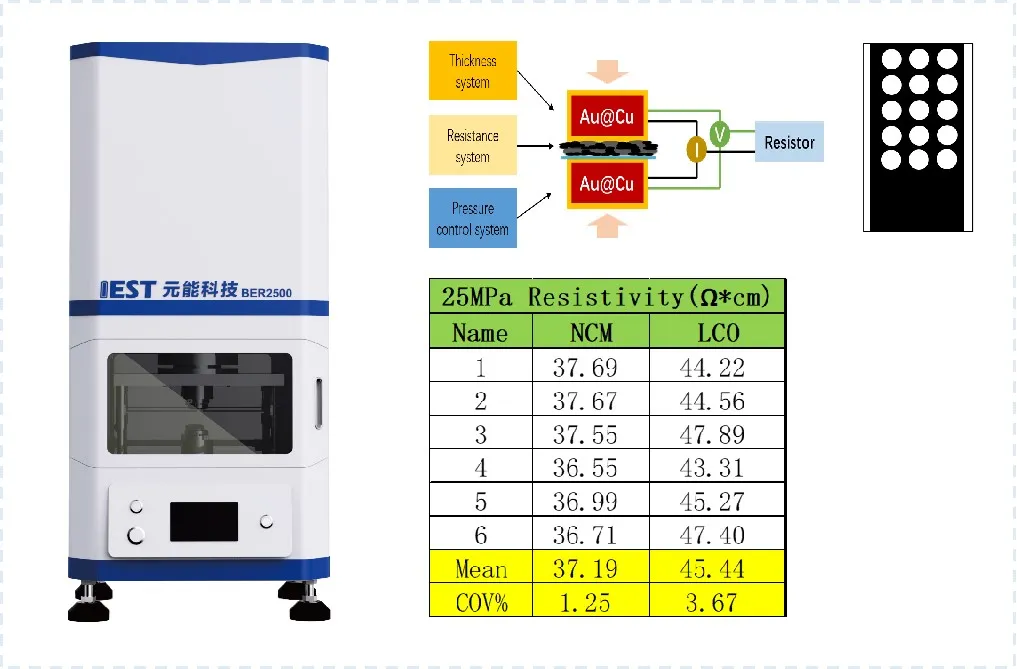
Electronic conductivity assessment of the dry electrode sheets is performed using the BER series instrument, which employs a dual-plane, pressure-controlled disk electrode method. This technique evaluates the through-plane electronic conductivity of the electrode/current collector assembly. It can be used to test electrode sheets prepared with different combinations of materials (cathode/anode materials, conductive agents, binders, current collectors) and optimize the process formulation. Furthermore, by measuring electronic conductivity at different points on the same electrode sheet, it effectively evaluates coating uniformity. This directly relates to the critical monitoring metric of film formation uniformity in the dry electrode process, as illustrated in Figure 4. This method is applicable for testing after both the dry coating and final calendering stages.
Figure 4. Schematic diagram of electrode coating uniformity evaluation principle
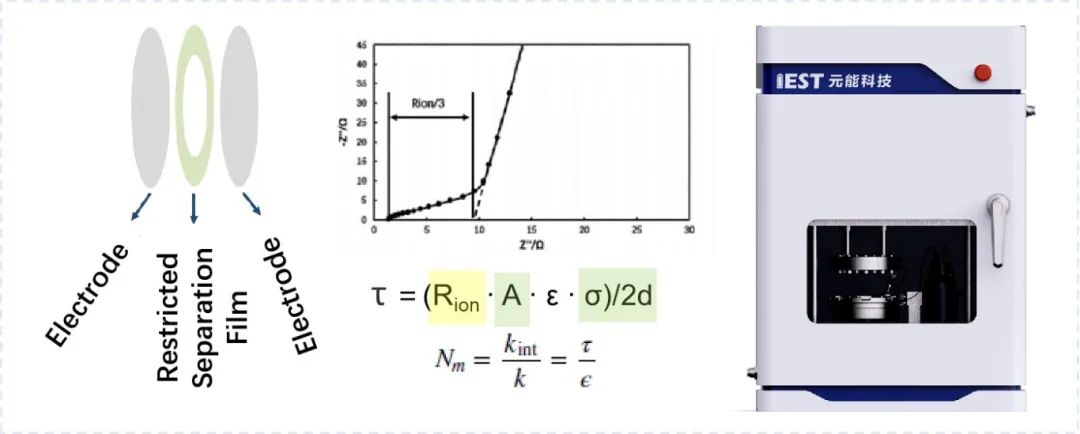
The evaluation of ion transport pathways in electrodes conducted at IEST Laboratory introduces the concept of tortuosity, which represents the degree of bending of the porous electrode transport paths. Tortuosity is another important parameter related to transport characteristics besides porosity. It can be used to characterize the electrolyte’s permeability and the migration rate of ions, thereby affecting the battery’s capacity and rate performance. Dry electrodes have significant advantages in post-coating processes. The electrode thickness is closely related to the effective ion transport path and rate performance. Effective evaluation of electrode-level tortuosity is crucial for predicting post-process electrochemical performance and assessing capacity performance.
Figure 5 illustrates the principle of electrode tortuosity evaluation based on the EIC series equipment. Electrochemical impedance spectroscopy (EIS) measurements are performed on symmetric cells assembled with electrodes, and the ion resistance (Rion) is calculated by combining the impedance values at high and low frequency ranges of the EIS curve. Further evaluation of tortuosity is conducted by incorporating parameters such as electrode area (A), electrolyte conductivity (σ), electrode thickness (d), tortuosity (τ), and MacMullin number (Nm). The introduction of Nm eliminates the need for porosity ε, directly assessing the effective transport pathway of lithium ions based on the MacMullin number concept.
Figure 5. Schematic diagram of electrode tortuosity measurement principle
5. Summary
The dry electrode process represents a promising frontier for green energy, offering advantages in both cost and battery performance, with potential applications in solid-state batteries and pre-lithiation. However, significant challenges remain in process manufacturing and industrialization. Researchers are focusing on the binding mechanism of dry-process binders, differences between binder types, long-term cycle performance at the cell level, and process stability assessment.
Researchers in the industry are particularly focused on understanding the bonding mechanism, differences in binder types, long-term cycling performance at the cell level, and stability assessment of the manufacturing process for dry electrodes. In the film formation stage of dry electrode processes, IEST has introduced series equipment such as PRCD, BER, and EIC to evaluate powder and electrode-level performance, aiding in process ratio optimization and process scheme modification.
In addition, for subsequent stages of the film formation process, IEST also conducts evaluations of electrochemical performance analysis, gas generation in cells, and expansion performance testing. These evaluations, along with others, provide new support and insights for scientific research and industrial applications.
6. References
[1] Zhang Y, Lu S, Wang Z, et al. Recent technology development in solvent-free electrode fabrication for lithium-ion batteries[J]. Renewable and Sustainable Energy Reviews, 2023.
[2] Landesfeind, Johannes,Hattendorff, et al. Tortuosity Determination of Battery Electrodes and Separators by Impedance Spectroscopy[J].Journal of the Electrochemical Society, 2016.
[3] Juarez-Robles D .Electroanalytical Quantification of Electrolyte Transport Resistance in Porous Electrodes[J].Journal of The Electrochemical Society, 2020, 167(8):080510 (16pp).
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.