-
iestinstrument
IEST Facilitates High-Nickel Cathodes Development for Stability and High-Rate Performance
1. Research Background
High-nickel cathode materials are widely recognized as a popular choice of cathode materials for high-energy-density lithium-ion batteries due to their high capacity and low-cost benefits. However, fast charging and long cycling at high voltage can trigger serious structural instability and stress-strain accumulation problems in high-nickel cathode, hindering its further commercialization.
2. Introduction of Research Work
Recently, Prof. Yuefeng Su, Researcher Lai Chen and Postdoctoral Fellow Jinyang Dong from Academician Feng Wu’s team at Beijing Institute of Technology (BIT) published a research paper entitled “Enhancing Chemomechanical Stability and High-Rate Enhancing Chemomechanical Stability and High-Rate Performance of Nickel-Rich Cathodes for Lithium-Ion Batteries through Three-in-One Modification”. In this study, a metal cation-induced three-in-one modification strategy was proposed to enhance the chemical and structural stability and high rate performance of the materials.W doping enhances the binding of oxygen on the surface layer of the materials and suppresses the oxygen loss induced by the transition from layered to rock salt phase during deep delithiation, thus enhancing the structural stability. The construction of a cationic mixed layer with appropriate thickness on the anode surface can improve the Li+ diffusion rate and mitigate the particle structure degradation. In addition, the Li2WO4 nano-coating layer reduces the side reaction between the active material and the electrolyte. It is important to conduct an in-depth study of the structural changes before and after cycling at different current multiplicities to better understand the multiplicity failure mechanism. These findings provide valuable mechanistic insights for the efficient utilization of high-nickel cathode materials and accelerate their large-scale industrialization and application.
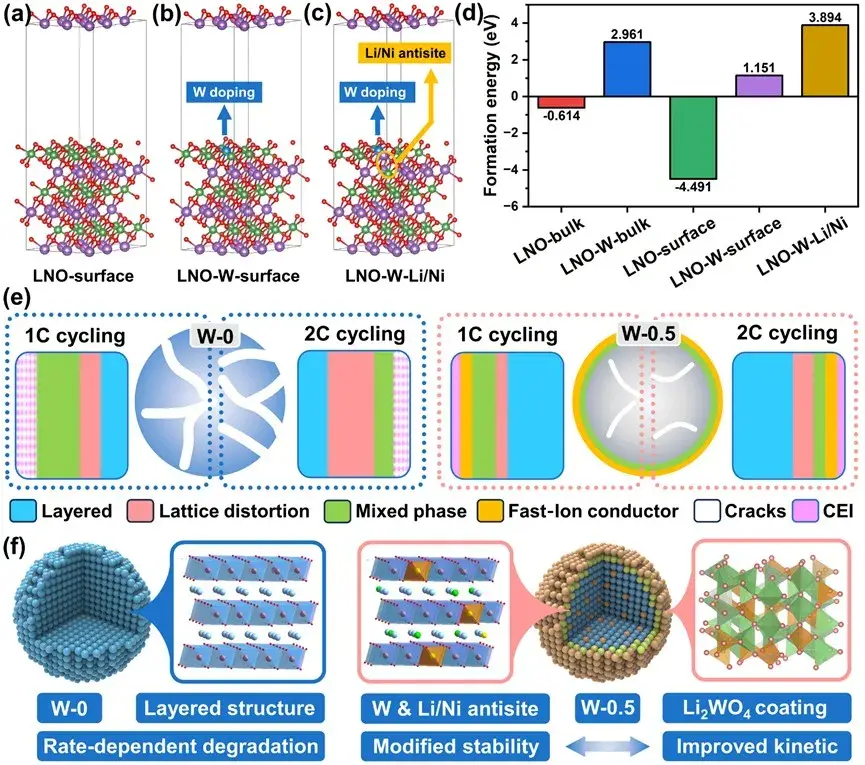
Figure. 1. Schematic diagram of hexavalent metal cation induced three-in-one modification mechanism and multiplicative failure mechanism
3. Core Content
3.1 Three-in-one mechanism to construct nano-surface layer
The inherent particle properties of the electrode materials determine the electrochemical performance of the battery, and in order to characterize the physicochemical features of the powder materials, the authors therefore used a IEST Powder Resistivity & Compaction Density Tester (PRCD3100) to measure the powder resistivity and conductivity. After modification, a large amount of residual alkali is consumed due to the formation of Li2WO4 capping layer on the surface of the material, which reduces the powder resistivity of the samples compared to the original material and thus enhances the electrical conductivity of the material; in order to further characterize the mechanical properties of individual particles, the authors used the high-precision pressure transducer and displacement control of the IEST Single-Particle Mechanical Properties Test System (SPFT2000) In order to further characterize the mechanical properties of single particles, the authors used the high-precision pressure sensor and displacement control of the SPFT2000 (IEST) to measure the mechanical properties of single particles, and it can be seen from the test that the three-in-one modification layer enhances the compressive strength of the material and reduces the displacement change when the material is crushed, which suggests that after modification, the material can withstand a greater internal stress to enhance the stability of the particle structure, and thus the compaction density of the material or electrodes can be further improved to increase the density of the capacity of the battery; after the PITT test, the reconfigured cationic hybrid layer and After the PITT test, the reconfigured cationic hybrid layer and Li2WO4 cladding layer significantly improve the Li+ transport efficiency of the material, which leads to the improvement of the cycling performance of the material under high current density. In summary, testing the key material performance parameters of individual cathode material particles helps us to deeply understand the mechanism of material performance changes.
Figure 2. Appearance of IEST Powder Resistivity & Compaction Density Tester(PRCD)
Figure 3. (a) IEST Single-Particle Mechanical Properties Test System (SPFT); (b) Test Mode with Controlled Displacement; (c) Bottom View of the Optical System
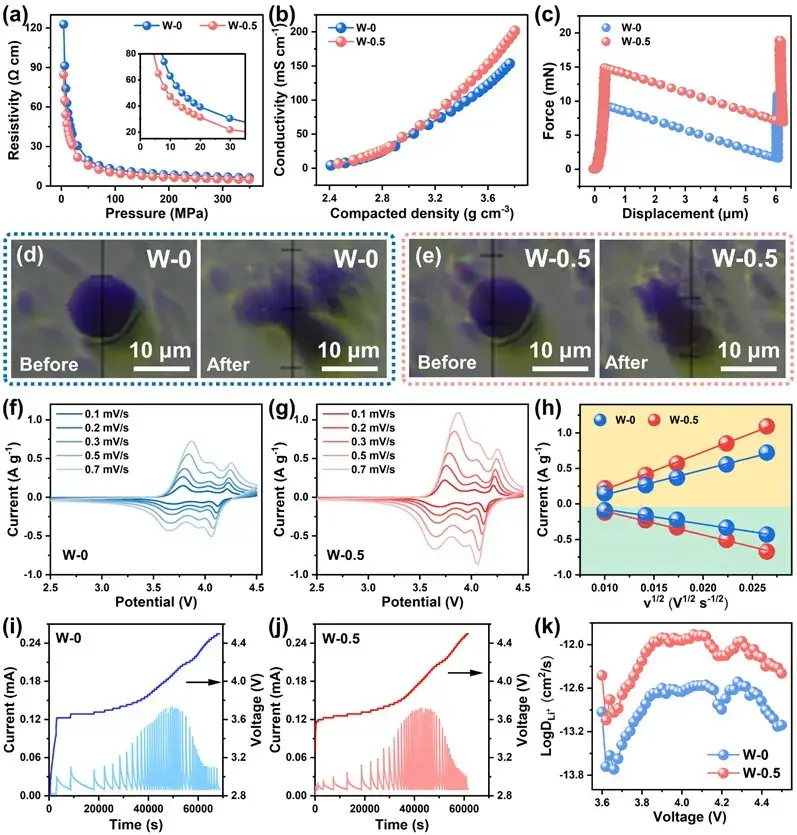
Figure 4. (a) Powder resistivity of W-0 and W-0.5 samples; (b) Electronic conductivity; (c) Single particle force test; (d) Optical photographs of W-0; (e) W-0.5 samples before and after single particle mechanical testing; (f) CV curves of W-0 and; (g) W-0.5 at 0.1-0.7 mV s-1; (h) Relationship between scan rate and peak current; (i) PITT test of W-0; (j) PITT test of W-0.5; (k) Corresponding Li+ diffusion coefficient
3.2 In-depth study of rate failure mechanisms
In-situ XRD, in-situ Raman, and in-situ DEMS tests were performed to investigate the modulation of lattice oxygen release and transition metal migration in the materials by DIEA engineering. In the in-situ XRD tests, the cell parameters of the material change slowly at high cut-off voltage, and the crystal structure is stabilized by strain mitigation. In-situ Raman tests were used to analyze the transition metal migration and structural degradation in the samples. The characteristic peaks at 500 cm-1 and 618 cm-1 in the modified samples showed small changes, while a new characteristic peak at 650 cm-1 in the pristine samples indicated that the material had a new spinel phase due to transition metal migration. In situ DEMS tests, on the other hand, show that the gas release of the material with a high-entropy surface layer is significantly suppressed at high pressures, suggesting a more stable lattice oxygen skeleton. Further DFT calculations were performed to provide theoretical support for the lattice oxygen release and transition metal migration, and the results show that the materials with high entropy surfaces still exhibit high oxygen vacancy formation energy and Mn migration energy barriers in the high delithiation state, which corroborates with the test results and indicates that the materials still exhibit more stable crystal structures under high pressure.
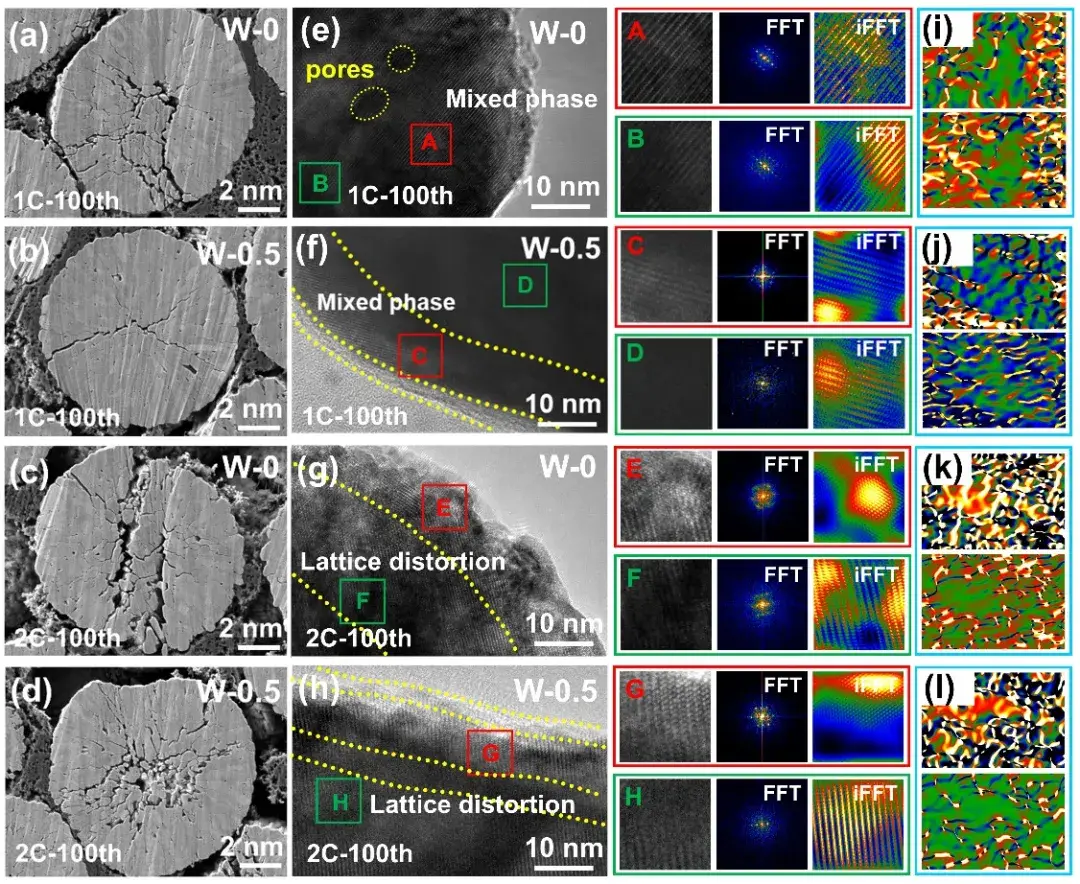
Figure 5. (a) SEM cross-sectional image of W-0 at 1 C; (b) SEM cross-sectional image of W-0.5 after 100 cycles at 1C; (c) W-0 at 2C; (d) SEM cross-sectional image of W-0.5 after 100 cycles at 2 C; (e) W-0 at 1C (f) W-0.5 at 1C; (g) W-0 at 2C; (h) SEM cross-sectional image of W-0.5 after 100 cycles at 2C; (i) W-0 at 1C; (j) W-0.5 at 1 C; (k) W-0 at 2C; (l) GPA analysis of W-0.5 at 2C
4. Summary
In this paper, a generalized hexavalent metal cation-induced three-in-one modification strategy is developed to cope with the stress accumulation of high-nickel cathode materials between long cycles at high-rate by enhancing the chemical and structural stability of high-nickel cathode materials. More importantly, this strategy not only strengthens the antioxidant performance of the material surface layer, but also reveals in depth the progressive structural changes of the material from the surface to the core under high multiplication rate. By systematically investigating the failure mechanisms under different charging and discharging multiplicities, we found that the stress conduction and phase transition behaviors inside the high-nickel cathode electrode significantly affect its overall performance in high-rate cycling. This finding not only provides a new perspective for understanding the material degradation mechanism under high-rate conditions, but also provides a scientific basis for future design strategies to regulate the material performance.
5. Original Article
Updated on Jan. 15, 2025
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.