-
iestinstrument
Surface Stress Analysis of Graphite Electrodes For Lithium-ion Batteries
1. Author Information and Article Abstract
Amartya Mukhopadhyay of Brown University of Engineering and Technology, USA, 2012, employs an optical stress sensing device for real-time (in-situ) measurement of stresses in thin films for the study of the development of irreversible stresses in graphite electrodes during the cycling process of lithium-ion batteries. The authors experimentally explored the electrochemical behavior of the graphite electrodes during the initial cycle, and deeply analyzed the evolution of the irreversible stress of the electrode during the process from the electrochemical perspective, the number of cycles, the thickness of the electrode, and the voltage (Irreversible Stress: the difference of the change of the stress during the lithium embedding/de-lithium removing cycle), which provides a new way of thinking for the study of the actual mechanical properties of lithium-ion batteries.
2. Measurement Principle
A parallel laser beam is focused on the backside of a quartz substrate (graphite electrodes carrier). The rigid substrate restricts the expansion and contraction of the in-plane dimensions of the active thin film (graphitic carbon in this case) during the lithium embedding/delithiation period, which causes the substrate/thin film system to bend, and the laser beam is deflected when it is reflected back from the substrate. As the quartz substrate deforms elastically, the stress in the film is proportional to the induced change in wafer curvature, and in situ measurement of stress development in the graphite electrodes can be achieved by monitoring the change in spot spacing of the deflected beam reflected from the backside of the substrate.
3. Measurement Information
3.1 Sample information
Graphitic carbon film (CVD C), a graphitic carbon layer prepared by chemical vapor deposition (CVD) and graphitization principles on a 250 nm thick quartz wafer with a diameter of 1 inch; and a 0.5 nm thick layer of Al2O3 deposited by atomic layer deposition (ALD) on top of the graphitic carbon layer, which blocks the formation of SEIs and embedding of solvated ions in the anode material, was used to study the effect on the electrochemical behavior and consequent stresses.
3.2 Experiments
A model cell of lithium metal was assembled with CVD C film in a customized electrochemical cell, subjected to constant current discharge/charge cycles, and the electrochemical behavior of the CVD C film and the ensuing stress changes were investigated by monitoring the curvature of the substrate (bending of the substrate/film system).
4. Result Analysis
4.1 Electrochemical Behavior During Initial Cycling
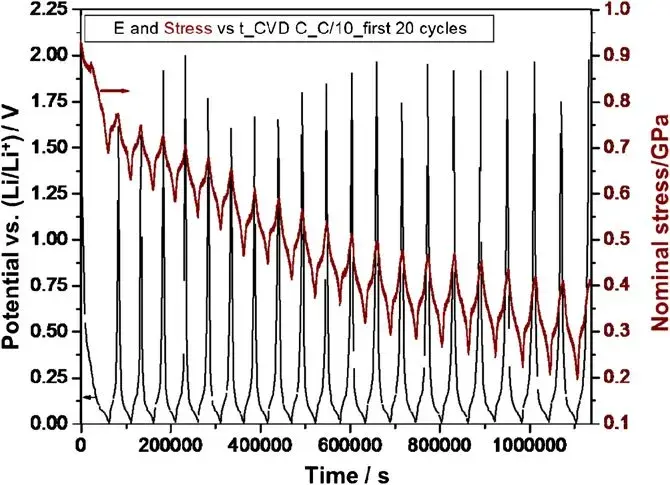
Figure 1. Changes in potential and stress of CVD C lithium insertion/delithiation cycle over time
Embedded huge Li capacity was observed during the embedded Li half-cycle in the first cycle, while the reversibility was relatively small during the de-Li half-cycle, removing some significant irreversible capacity loss after the first cycle, most of the capacity was reversible and consistent for different cycle rates. The graphite electrodes (CVD C) was repeatedly charged with Li to near theoretical capacity at electrochemical cycling rates of C/5, C/10, and C/20, and the similarity in capacity for the three rates indicates that Li diffusion is not rate-limited in this material. Furthermore, after 50 cycles, there was no capacity decay or any significant macro- and microstructural damage, and the voltage data were smooth.
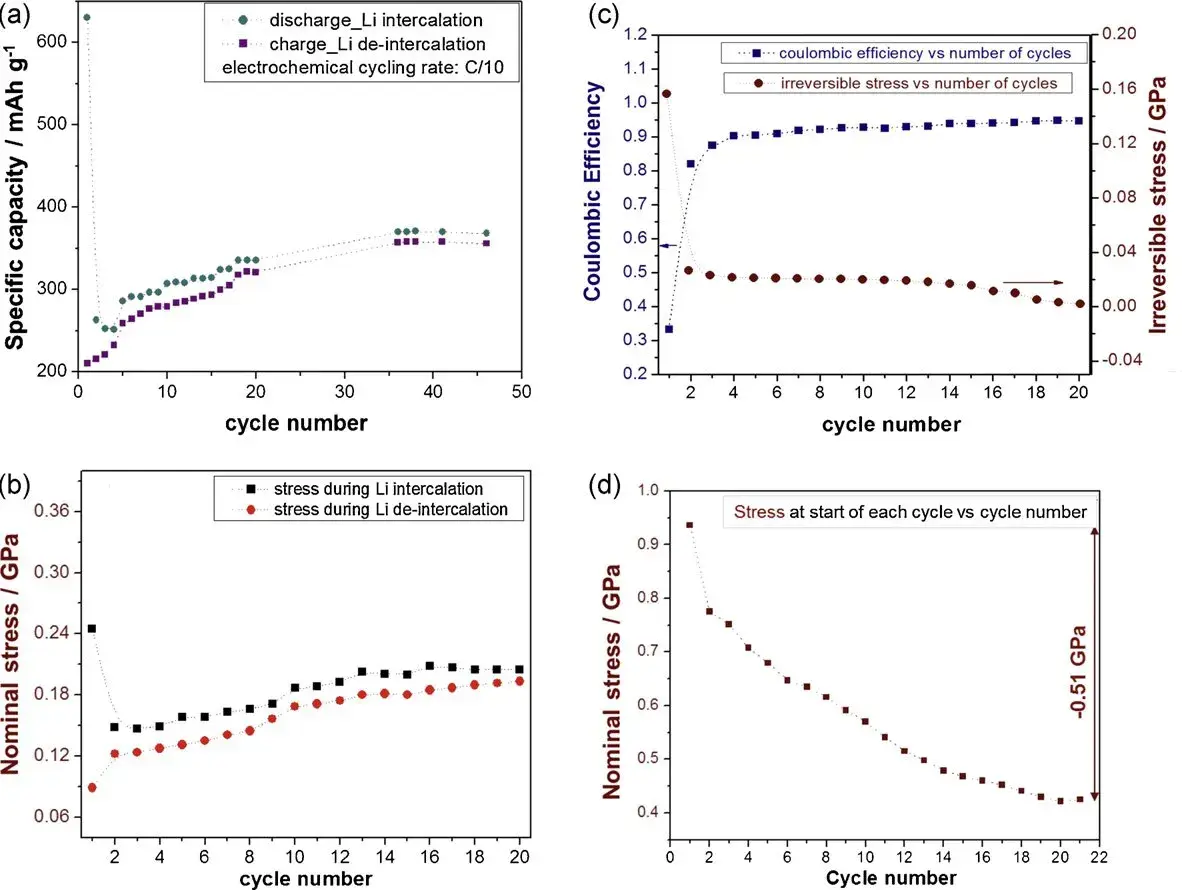
Figure 2. (a) Variation of Li capacity with the number of cycles during half cycles of Li embedding and delithiation; (b) Nominal stresses measured during half cycles of Li embedding and delithiation; (c) Coulombic efficiencies and irreversible stresses; (d) Stresses present in the sample at the beginning of each cycle;
The irreversible capacity, i.e., the difference in Li capacity for the Li embedding/delithiation process, is described by the Coulombic efficiency (CE). The cumulative irreversible stress in the initial cycle, mentioned in the article, is two times higher than the stress induced by the reversible stress, and the surface effect of the irreversible stress (film thickness effect) suggests that the irreversible component is mainly determined by one or more phenomena occurring near the surface of the film. The high capacity and consequent high stress recorded during the embedded lithium half-cycle in the first cycle and the relatively low capacity and stress reversal during the delithiation lead to a large difference between the capacity as well as the stress during the first two half-cycles, with very low Coulombic efficiency (CE) values in the first cycle. The correlation between electrochemical behavior and stress development during the first 20 cycles and the fact that the irreversible stresses are only from surface phenomena suggest that a large amount of irreversible stresses are directly related to the formation of the SEI layer.
The stress evolution in the initial cycle is also shown in more detail in Figures 2b-2d, the author designed a graphitic carbon film with a lattice coefficient parallel to the substrate so that the stress development is only related to the change of the film. During discharge (half cycle of lithium insertion), a net compressive stress will be generated, and then it will be reversed during charging (half cycle of lithium removal). In the first lithium insertion cycle, a large part of the compressive stress is not reversed (equivalent to the initial residual stress). The magnitude of the irreversible stress is significantly reduced in the second cycle, and this trend continues until the difference between the stresses of the two half cycles in the later period is negligible, the stress development during the delithiation/intercalation process is almost completely reversed. In this experiment, The maximum in-plane stress change caused by the insertion of lithium is about -0.25 GPa (reversible stress, Figure 2b), the irreversible net compressive stress formed in the initial cycle is much higher (about -0.5 GPa, see Figure 2d). The author believes that this irreversible stress is an important part of the characteristics of feedback materials.
4.2 Sources of irreversible stress
4.2.1 Surface effects of irreversible stresses
The authors verified by assumptions that the irreversible stress originates from the surface layer of the graphitic carbon layer, independently of the thickness of the CVD C-film. By varying the thickness study, if the irreversible stress appears inside the film, the corresponding stress thickness is proportional to the film thickness; conversely, if this contribution originates from the surface layer of the graphitic carbon layer, the stress thickness is independent of the film thickness.
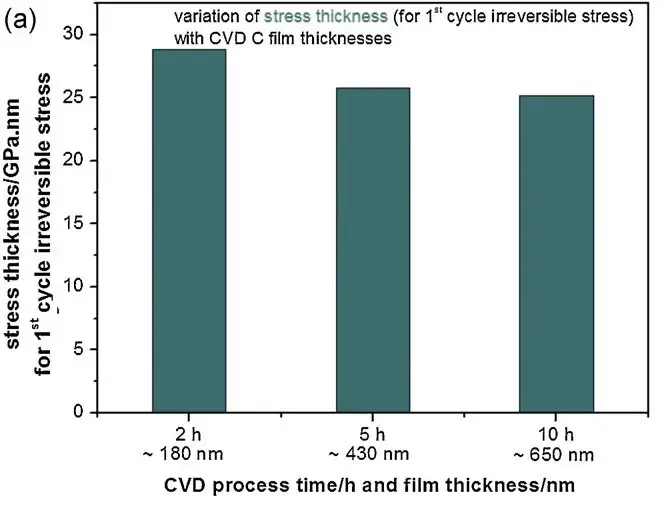
Figure 3. (a) Actual stress thickness for irreversible stresses with different thicknesses of film
4.2.2 Stress changes under different voltages
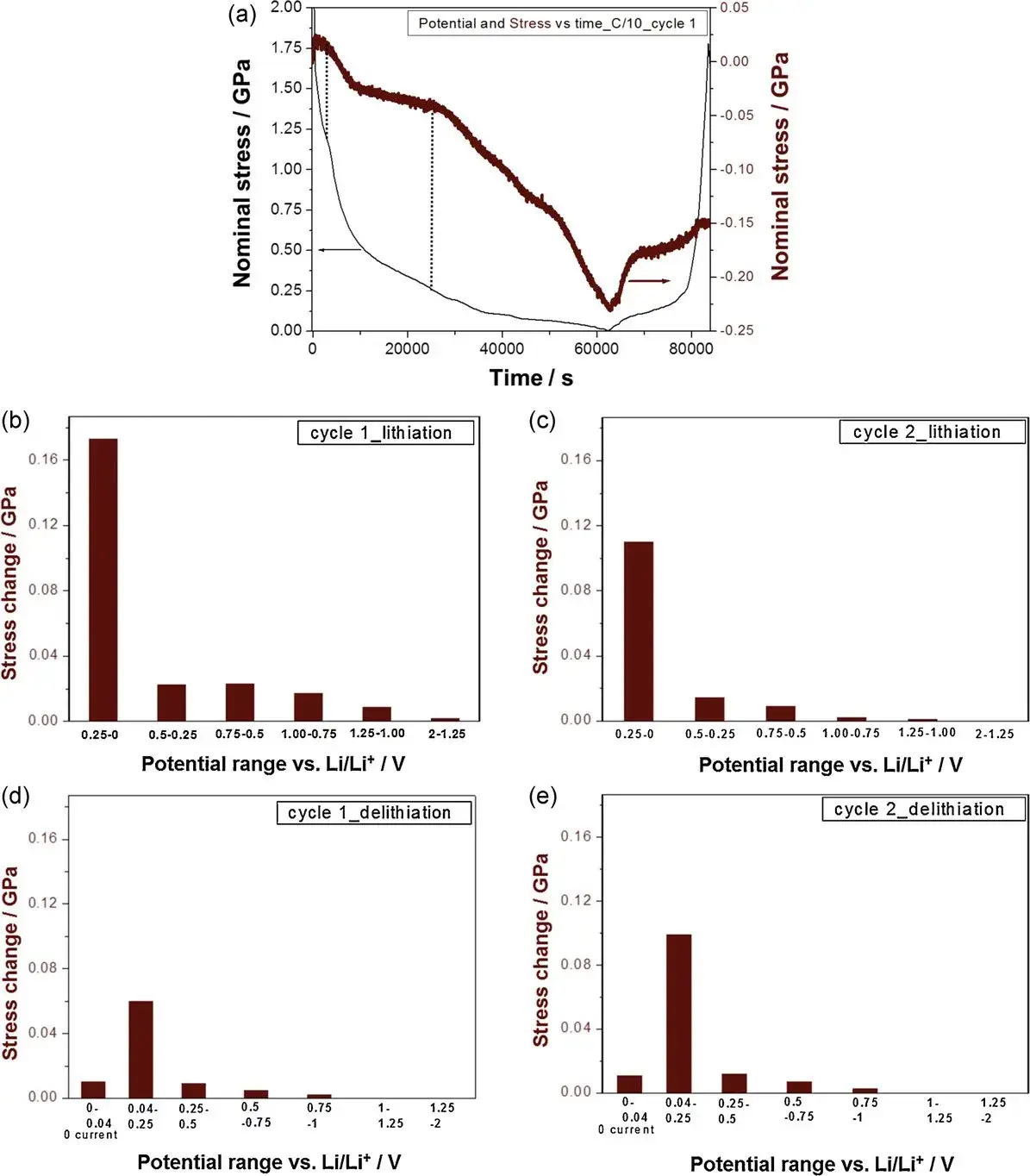
Figure 4. (a) Potential and nominal stress (corresponding to the residual stress of the deposited film) evolution with time for the first cycle;
(b)-(e) Stress evolution in different potential ranges (embedded lithium/delithium half-cycle)
It is known that below ~0.25 V the actual Li embedding is predominant and the rapid change in compressive stress can be seen in the potential, and once the potential reaches ~1.0 V, a protective SEI layer is formed, which almost completely suppresses the solvated ionic co-embedding. Figures 4b-e summarize the stress contributions measured in different voltage ranges during lithium embedding and delithiation in cycles 1 and 2. The authors show that most of the compressive stresses in the lithium embedding half-cycle occur below 0.25 V, the stresses occur for actual lithium ion embedding, and that this voltage range is also accompanied by the ongoing formation of the SEI layer during the first few cycles, i.e., the cause of the small amount of irreversible compressive stresses observed after the second cycle is also the formation of the SEI layer; whereas, any stresses at higher potentials (> 0.25 V ) in the first cycle are directly related to SEI formation or possible co-embedding of solvated ions.
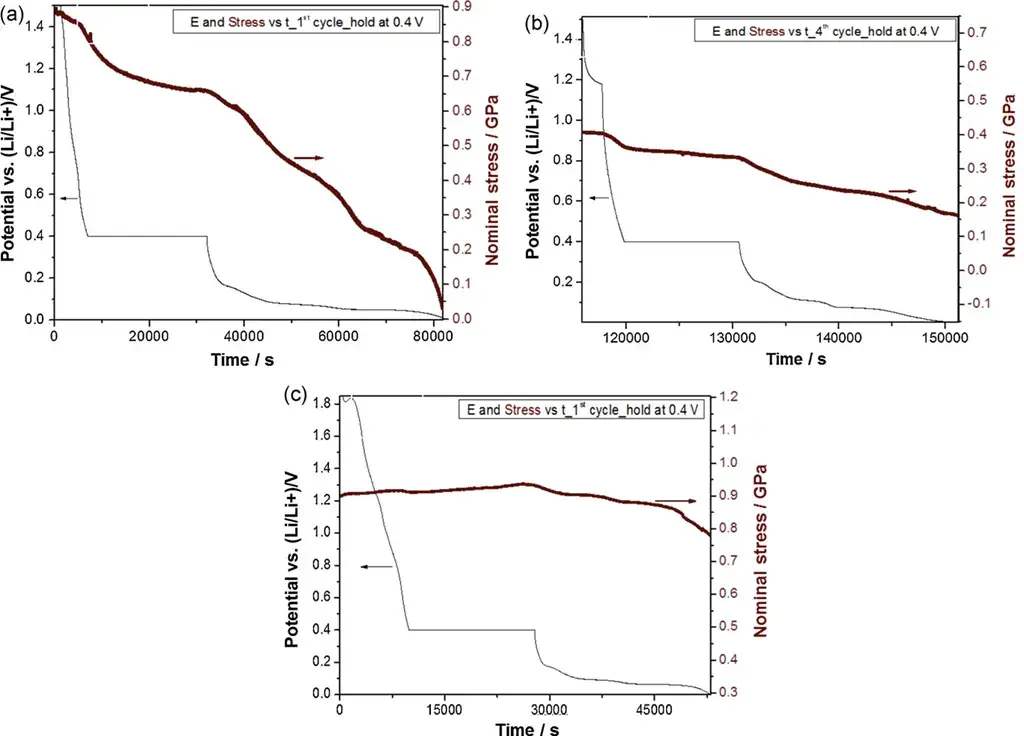
Figure 5. (a) Stress variation in the first embedded lithium half-cycle; (b) Stress variation in the second embedded lithium half-cycle; (c) Stress variation in the first embedded lithium half-cycle (Al2O3-coated CVD C-sheet)
Figure 5a stress variations are interpreted as Li embedding + SEI formation + embedding of solvated ions; Figure 5b, as Li embedding + continued slow formation of passivated SEI; and Figure 5c, as actual Li embedding in graphite (with the Al2O3 coating blocking SEI formation), which further confirms that the main source of irreversible stress is SEI formation.
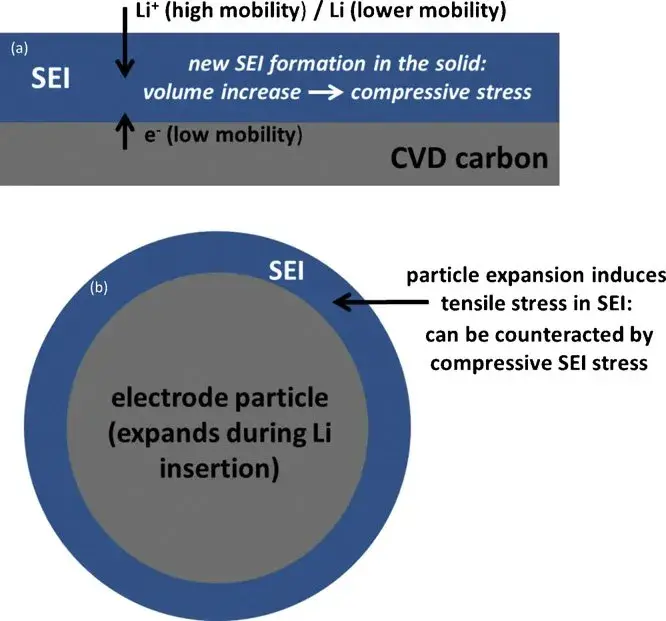
Figure 6. (a) Schematic representation of the formation of the SEI film and accompanying stresses on the thin-film graphite electrodes; (b) expected behavior in the graphite particles and the surrounding SEI layer
6. Summary
In this research, an optical stress sensing device is used to measure the stress in the film in real time (in-situ). The first few electrochemical cycles of graphite electrodes against Li metal are reported for the first time. Discuss the evolution of the huge irreversible compressive stress generated in the thin-film graphite electrodes. The relationship between the irreversible stress and the formation of SEI is determined from multiple angles, which provides important guidance for the study of the electrochemical behavior and actual mechanical properties of lithium-ion batteries.
7. References
Mukhopadhyay A, Tokranov A, Xiao X, et al. Stress development due to surface processes in graphite electrodes for Li-ion batteries: A first report[J]. Electrochimica Acta, 2012, 66(none):28-37.
8. IEST Related Test Instruments Recommendations:
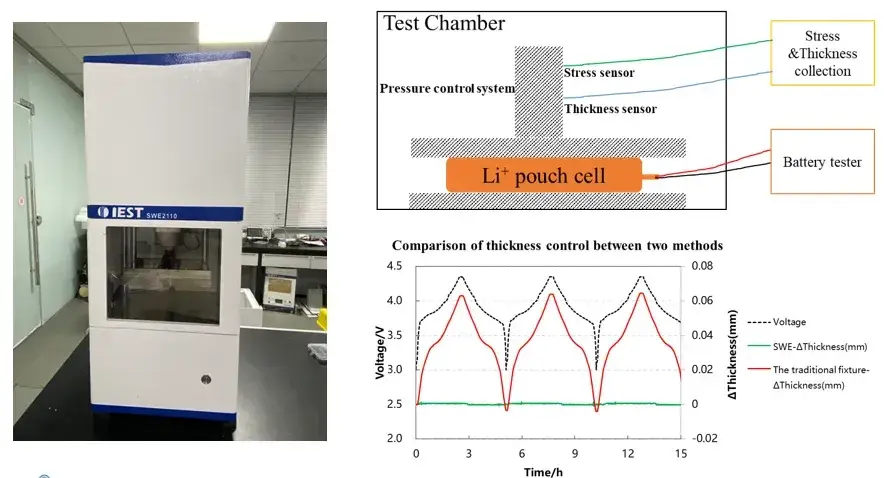
SWE series in-situ swelling analysis system (IEST):
1. Integration of multiple in-situ cell characterization methods (stress & swelling thickness): Measure the swelling thickness and swelling force during the charging and discharging process of the cell at the same time, and quantify the changes in the swelling thickness and swelling force of the cell;
2. More detailed and stable testing system: utilizing a highly stable and reliable automated regulation platform, equipped with high precision thickness measurement sensors and pressure regulation system, the relative thickness measurement resolution is 0.1µm, realizing the long cycle monitoring of the long-term charging and discharging process of the battery cell;
3. Diversified environmental control and testing functions: SWE series equipment can adjust the temperature of the charging and discharging environment, which is helpful for the study of the swelling behavior of the electric core under high and low temperature conditions; in addition to the conventional thickness and pressure testing, it can also realize the testing of parameters such as the swelling force of the electric core, the compression modulus, and the compression rate.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.




