-
iestinstrument
Characterization Of High Nickel Ternary Gas Production Behavior
1. Preface
Among the many cathode materials, the high nickel material LiNixM1-xO2 (M = Mn, Co, Al, etc.) exhibits high energy density as well as good cycle life. However there is a decreasing trend in the market share of high nickel cathodes compared to LiFePO4 (LFP). One of the main reasons for this phenomenon is the poor safety performance of high nickel cathode at high state of charge (SOC) compared to LFP. Specifically, the poor safety performance of cathode is mainly due to two aspects: the exothermic reaction of the cathode in the charging state and the resulting thermal runaway of the battery, and the gassing reaction between the electrolyte and the cathode and the resulting increase in the internal pressure of the battery.
The gas production mechanism of high nickel cathode materials mainly consists of two aspects [1]: on the one hand, it is the decomposition of residual lithium (such as LiOH, LiHCO3, and Li2CO3) on the surface of the material, and the cause of residual lithium is mainly the instability of Ni3+ in air, and CO2 and H2O can react with the cathode to promote the formation of residual lithium and the NiO layer of rock salt; on the other hand, compared with the residual lithium gas production, the majority of the gases are generated from the oxidative decomposition reaction of the electrolyte. electrolyte oxidative decomposition reaction. For this reason, we can monitor the gas production behavior in-situ with the in-situ battery gassing volume analyzer (GVM2200) from IEST.
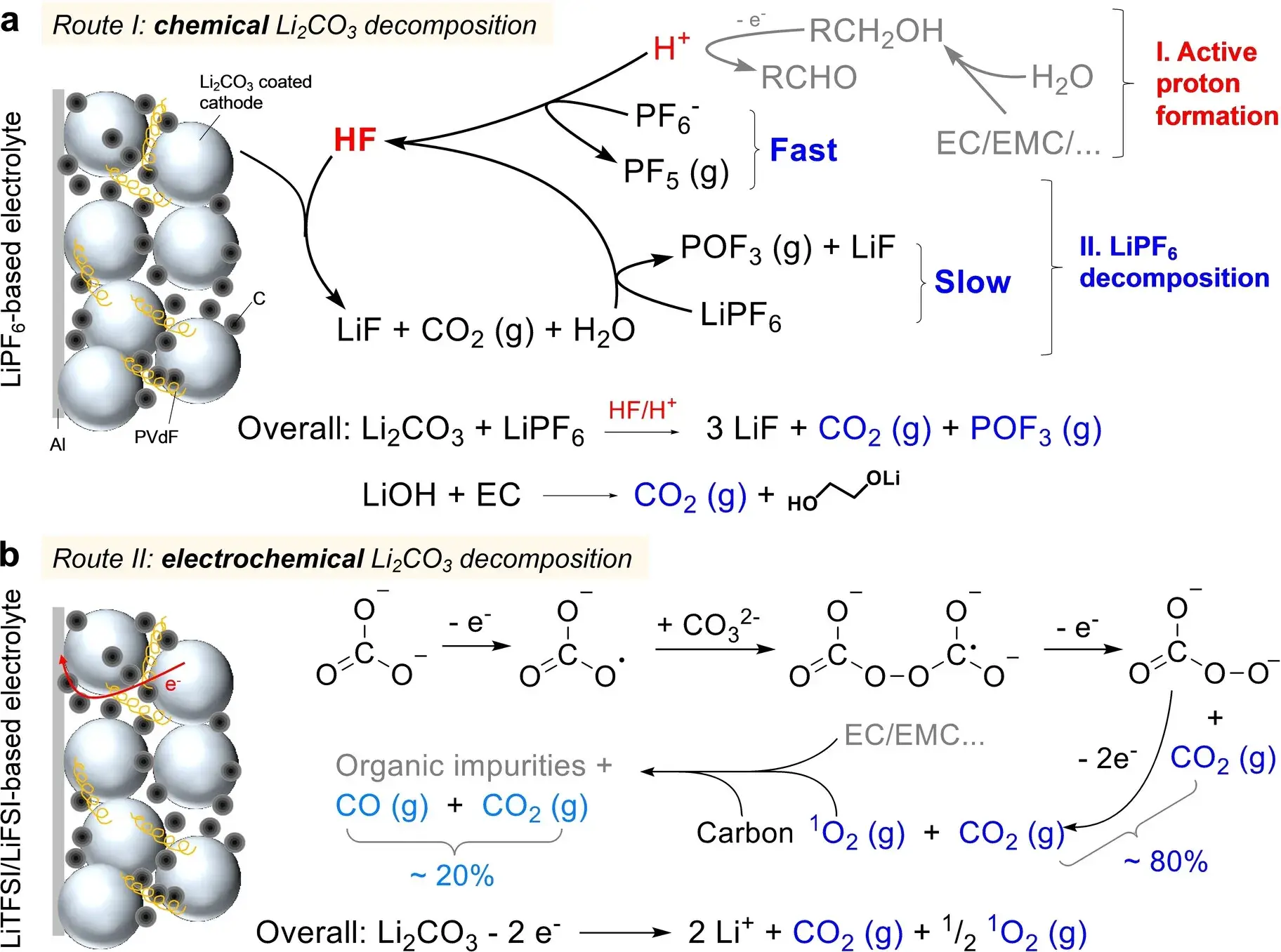
Figure 1. Schematic representation of the (a) chemical decomposition pathway and (b) electrochemical decomposition pathway for Li2CO3 and LiOH ¹
2. Test Information
2.1 Experimental instrument
In-situ battery gassing volume analyzer, model GVM2200.
 Figure 2. In-situ battery gassing volume analyzer, GVM2200
Figure 2. In-situ battery gassing volume analyzer, GVM2200
2.2 Cell information
Table 1. Cell Information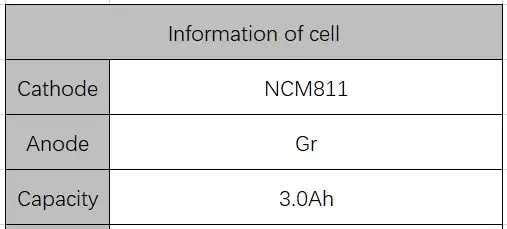
2.3 Testing process
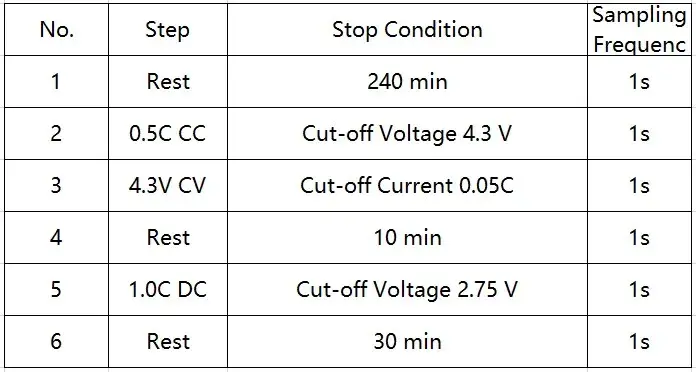
Table 2: Charging and discharging steps
3. Experimental process and analysis
Two NCM811 materials with the same nickel content but prepared by different processes were selected, and the same anode materials and electrolyte were used to make soft-cells A and B according to the same core process, and the GVM2200 equipment was turned on, the test temperature was adjusted to 25°C, and the gas production behaviors of the cells were monitored with the two channels in situ.
The first cycle is shown in Figure 3: with the change of voltage, the volume change of the cells can be seen in situ, and the volume change of the two cells shows the characteristic curve of high nickel ternary/graphite (for details, please refer to the application case: “Analysis of Swelling Behavior in NCM Cathode Materials”). In addition, the decrease in volume at the end-of-discharge shelf stage may be related to the elimination of the uneven distribution of lithium concentration and the elimination of the internal temperature gradient at the end of the cell discharge (for details, please refer to the application case: “The Effect of Charge-discharge Rate On Lithium Cobalt Oxide(LCO)/Graphite Volume Expansion In Shelving Stage”).
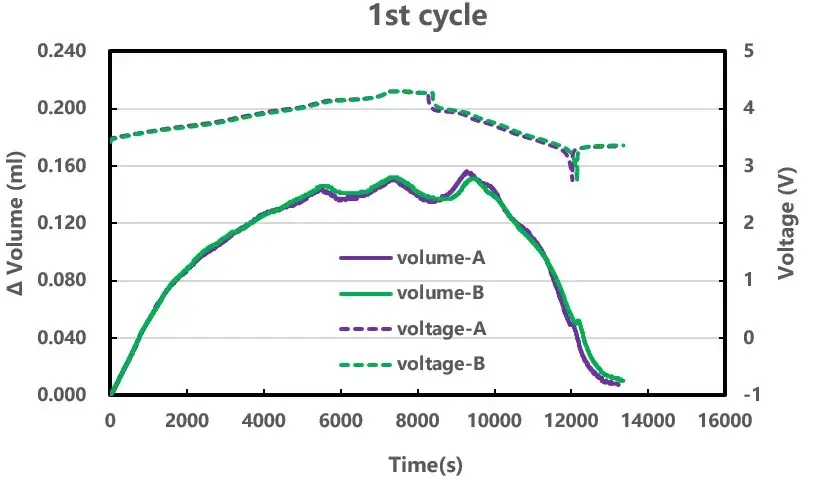
Figure 3. Cell 1st cycle volume and voltage change curve
The volume change behavior of the two cores in a single cycle did not have a significant difference, and then increase the number of cycles of the core, the results are shown in Figure 4. With cycling, the volume of the battery cell shows a wave-type change, which may be related to the gas produced by the battery cell in the cycling process and then re-involved in the reaction to be absorbed ², in addition to the reversible volume change of the graphite negative electrode. Throughout the life cycle of the core, there are more factors that cause the volume change of the core, such as the phase change of the active material de-embedded lithium, the SEI film growth, and the gas production. In order to minimize the influence of coupling factors on gas production, this experiment chooses room temperature and few cycle experiments, and compares the volume difference of the same state, so as to identify the change of gas production in the battery cell, as shown in Figure 5. The different states of charge (SOC) all reflect that the gas production volume of cell A with cycling is smaller than that of cell B, and this gap shows an increasing trend with the increase of the number of cycling laps, which indicates that the material preparation process A is better than that of process B. The gas production volume of cell A with cycling is lower than that of cell B with cycling.
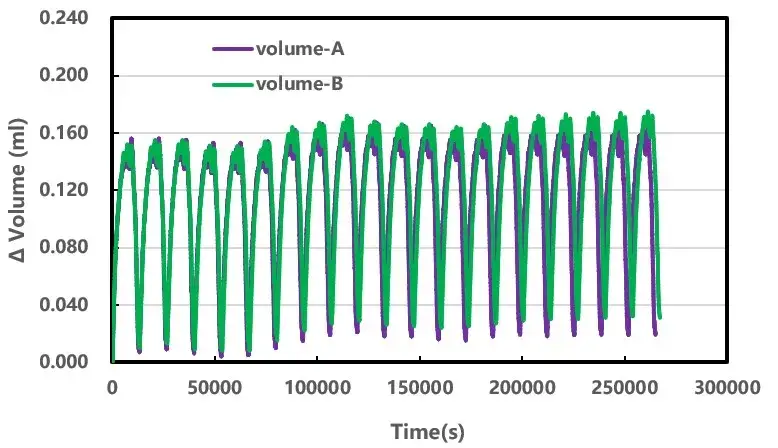
Figure 4. Cell volume and voltage variation curves
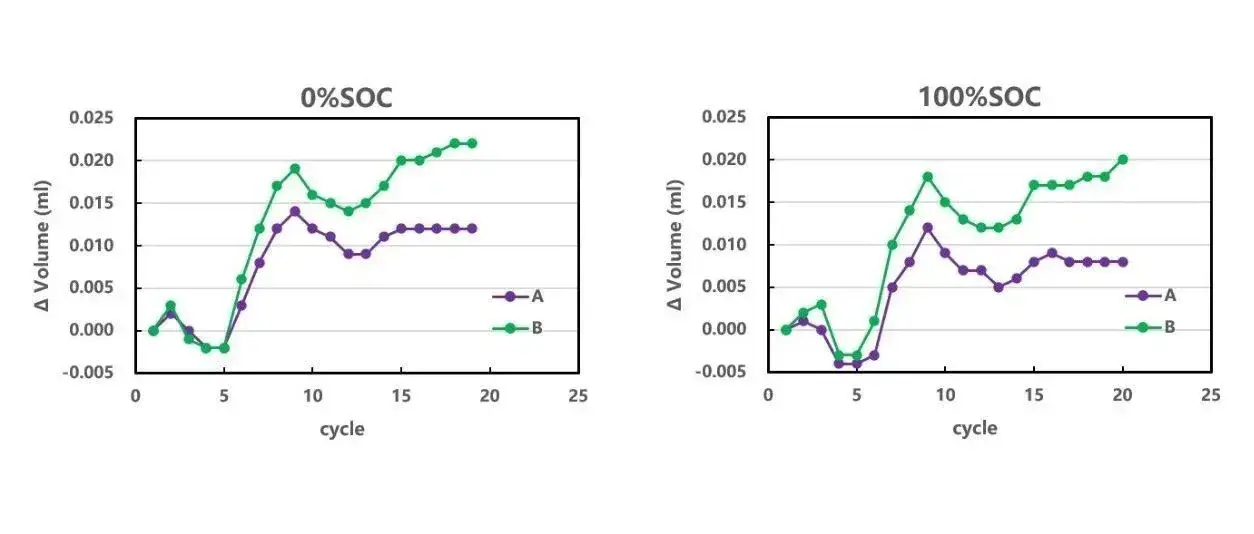
Figure 5. Variation of gas production of the cell with cycling
4. Summary
This experiment quantitatively evaluated the effect of different modification conditions of high nickel ternary materials on the gas production of the core using the in-situ gas production volume monitor (GVM2200) of IEST, which can provide a characterization method to evaluate the gas production of high nickel materials for the related technicians, which can help the development of high nickel materials as well as the improvement of the process, so as to prepare the materials with more excellent performance.
5. References
[1] Zehao Cui and Arumugam Manthiram*, Thermal Stability and Outgassing Behaviors of High‐nickel Cathodes in Lithium‐ion Batteries, Angew. Chem. Int. Ed. 2023.
[2] Xiaoyan Hu; Gas production study of high nickel/silicon-carbon lithium-ion batteries under high temperature conditions [J]; Battery Industry;2024
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.


