-
iestinstrument
Three Stages of Lithium Plating and Dynamic Evolution Processes in Pouch C/LiFePO4 Batteries
First Authors: Ying Lin, Wenxuan Hu
Corresponding Author: Yong Yang
Affiliation: Xiamen University
Equipment Used: IEST Silicon-Based Anode Swelling In-Situ Screening System(RSS1400), IEST In-Situ Cell Swelling Testing System(SEW2100)
Abstract
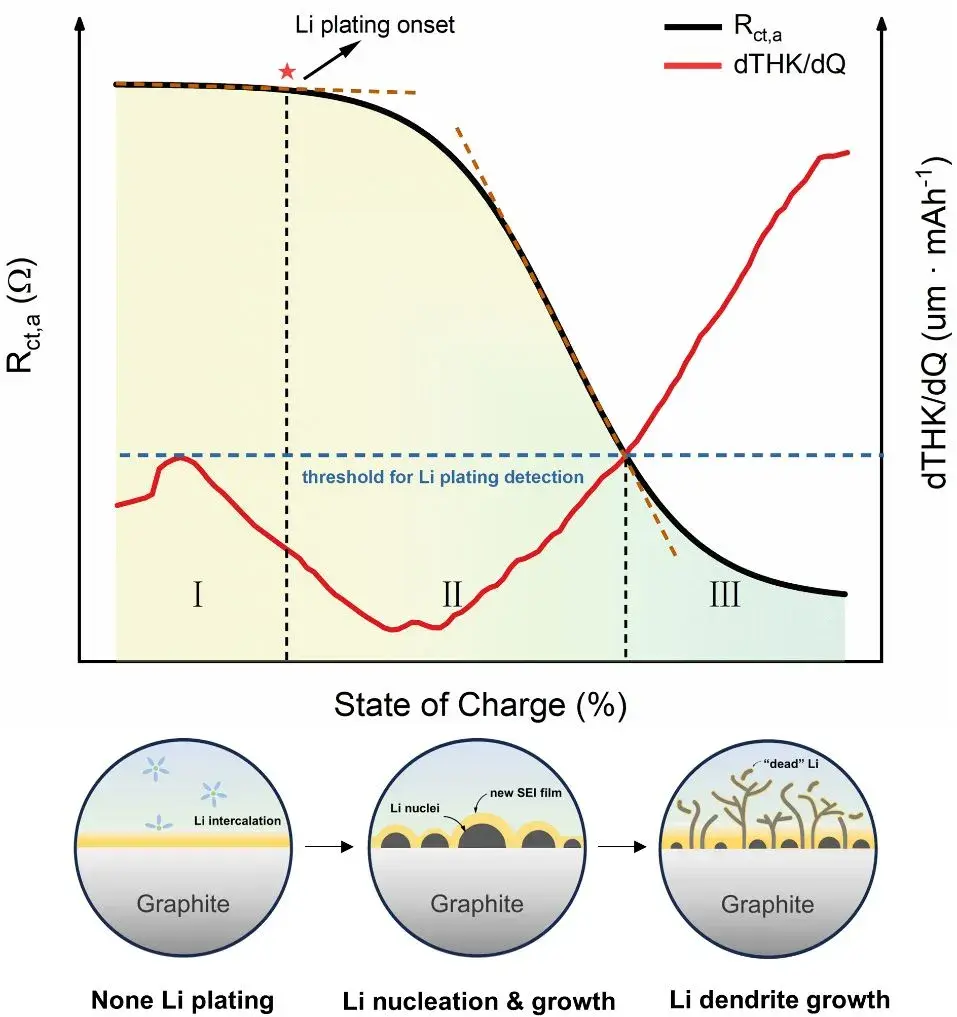
Lithium plating on graphite anodes during fast charging or low-temperature operation presents a major safety and lifetime risk for lithium-ion batteries. This study integrates in-situ dynamic electrochemical impedance spectroscopy (DEIS) with high-precision thickness monitoring to resolve the dynamic evolution of lithium plating in commercial pouch C/LiFePO₄ cells. DEIS-derived negative-electrode charge-transfer resistance (Rct,a) reveals a reproducible three-stage pattern—(I) graphite lithiation, (II) lithium nucleation/growth, and (III) dendritic growth—while in-situ thickness (dT/dQ) provides complementary macroscopic evidence of plating severity. Correlative ex-situ mass spectrometry titration (MST) and SEM validate the electrochemical-mechanical descriptors. The combined impedance–thickness approach enables earlier detection, staging, and quantification of lithium plating and informs mitigation strategies for BMS and electrode design.
1. Research Background
Lithium plating—metallic Li deposition on graphite—occurs under extreme conditions (e.g., fast charging, sub-ambient temperatures) and can form electrically isolated “dead lithium,” accelerate SEI buildup, and produce dendrites that risk short circuits and thermal runaway. Existing detection methods often identify plating onset but lack resolution to describe its subsequent morphological evolution. Here we apply a multidimensional, in-situ methodology combining DEIS and sub-micron thickness monitoring to (1) detect plating onset with high SOC resolution, (2) classify deposition states, and (3) link plating stages to capacity fade and safety risk metrics.
2. Work Overview
Recently, Professor Yong Yang team at Xiamen University comprehensively studied the evolution process of Lithium plating on graphite surfaces in graphite/LiFePO4 pouch batteries under harsh conditions (low temperature/room temperature fast charging) using a combined analysis method of in-situ dynamic electrochemical impedance spectroscopy (DEIS) and thickness measurements. This work expands the application of impedance spectroscopy and thickness measurement in detecting Li plating. Researchers found that the anode charge transfer resistance Rct,a exhibits a three-stage variation pattern as Li plating progresses. Combined with mass spectrometry titration (MST) and scanning electron microscopy, they confirmed that these three stages correspond to different Li plating evolution processes: non-plating, lithium nucleation & growth, and dendrite growth. The study also extensively analyzed the effects of lithium plating and different lithium deposition states on battery capacity degradation. This research titled “Unveiling the Three Stages of Li Plating and Dynamic Evolution Processes in Pouch C/LiFePO4 Batteries” was published in the prestigious journal “Advanced Energy Materials”.
3. Experimental Overview
2.1 Cell chemistries and preparation
Commercial pouch cells with graphite anodes and LiFePO₄ cathodes (C/LiFePO₄) were used. For decoupling electrode contributions in thickness signals, LTO (zero-strain) was used in dedicated LTO//LFP and LTO//Graphite assemblies.
2.2 In-situ instrumentation and measurement strategy
-
DEIS (dynamic EIS): A continuous AC perturbation (50 kHz → 5 Hz) was applied during charging. Low-frequency data were de-emphasized to obtain EIS spectra every 33 s (<1% SOC resolution). DRT (distribution of relaxation times) analysis extracted the negative-electrode charge transfer resistance Rct,a as a dynamic descriptor.
-
Thickness monitoring: IEST RSS1400 provided high-resolution (0.1 µm) thickness traces for rapid decoupling experiments; IEST SEW2100 recorded real-time pouch cell thickness under controlled preload (285 kg) and temperature (0°C) to quantify dT/dQ.
-
Ex-situ validation: Mass spectrometry titration (MST) quantified “dead lithium” and SEI species. SEM/optical microscopy characterized deposited lithium morphology at representative stages.
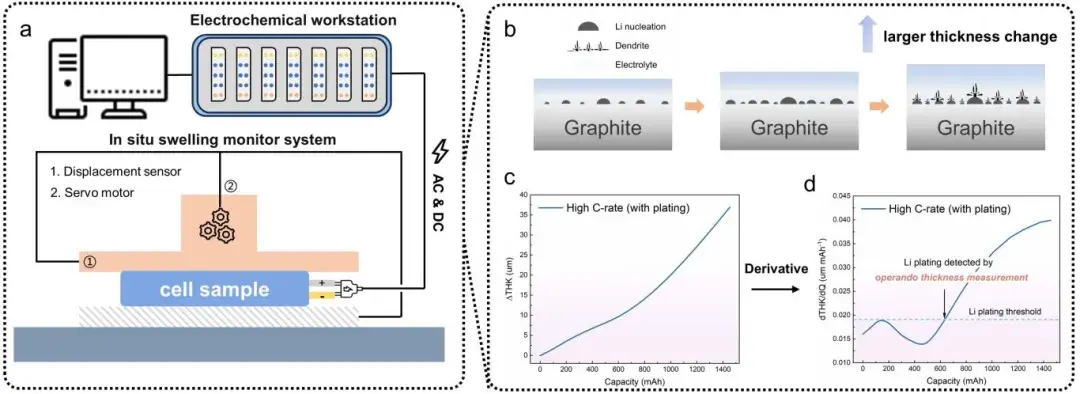
Figure 1. Schematic diagram of the in-situ dynamic electrochemical impedance-thickness measurement device used for Li plating detection experiments (consisting of an electrochemical workstation and an in-situ expansion test system), and a method for detecting the occurrence of Li plating by thickness measurement.
2.3 Test matrix
Cells were charged at multiple C-rates (0.1C, 0.2C, 0.5C at 0°C and room temperature high-rate tests) to induce a spectrum of plating behaviors. Incremental capacity analysis (ICA) and differential OCV (dOCV) were used to cross-validate plating indicators.
3. Key results
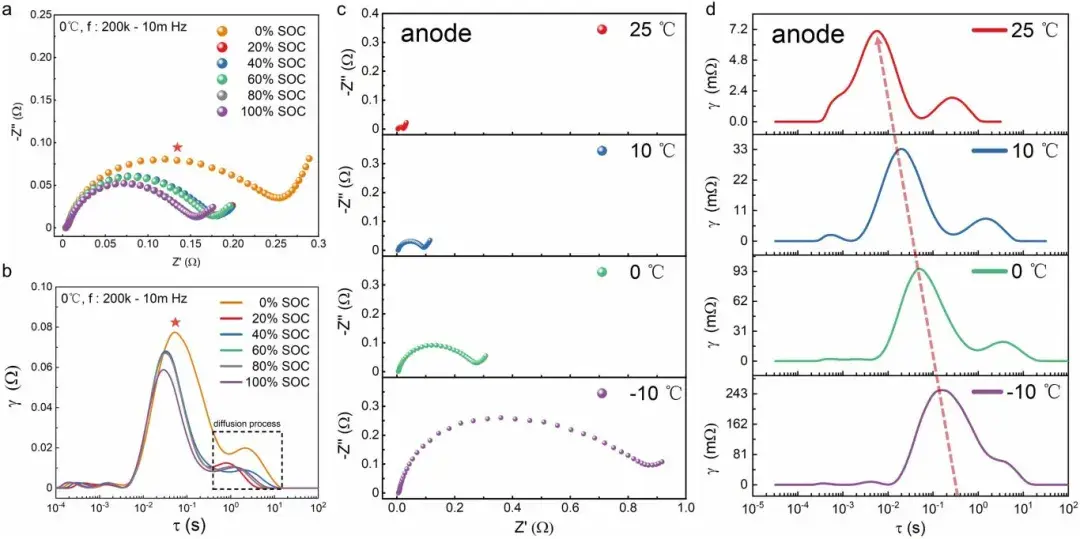
Figure 2. Shows the negative electrode EIS spectra (Nyquist plot and DRT) at different state of charge (SOC) and temperatures.
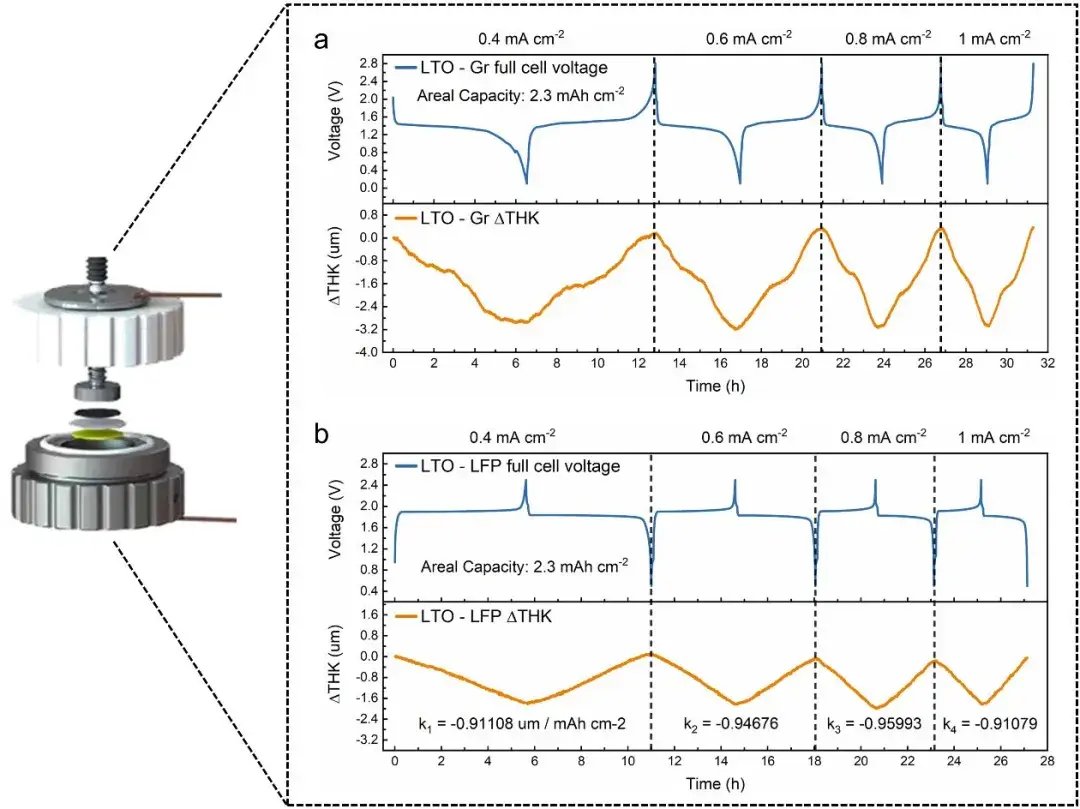
Figure 3.Illustrates the decoupling of graphite and LiFePO4 expansion behaviors using LTO.
3.1 DEIS identifies a three-stage Rct,a evolution during charging
DEIS/DRT analysis of Rct,a revealed a reproducible three-stage profile under plating-prone conditions:
-
Stage I — Linear decrease: Rct,a decreases gradually, consistent with normal graphite lithiation kinetics.
-
Stage II — Accelerated decline (nucleation/growth): Rct,a falls more rapidly; DRT indicates the emergence of a plating-related relaxation mode consistent with nascent Li nucleation and small aggregate growth.
-
Stage III — Plateau (dendrite growth): Rct,a reaches a near-steady plateau despite continued charging, interpreted as a surface increasingly dominated by metallic Li coverage and dendritic morphology.
Stage II represents a transitional region where insertion and plating coexist; Stage III is dominated by plating reactions.
In conclusion, both impedance and thickness measurements can detect the process of lithium plating, but they provide different types of information and potential physical significance. They complement each other and cross-validate findings. Electrochemical information (Rct,a) and structural information (dT/dQ) together form multidimensional descriptors to accurately determine the state of lithium deposition, facilitating a more comprehensive understanding of the evolution process of lithium plating.
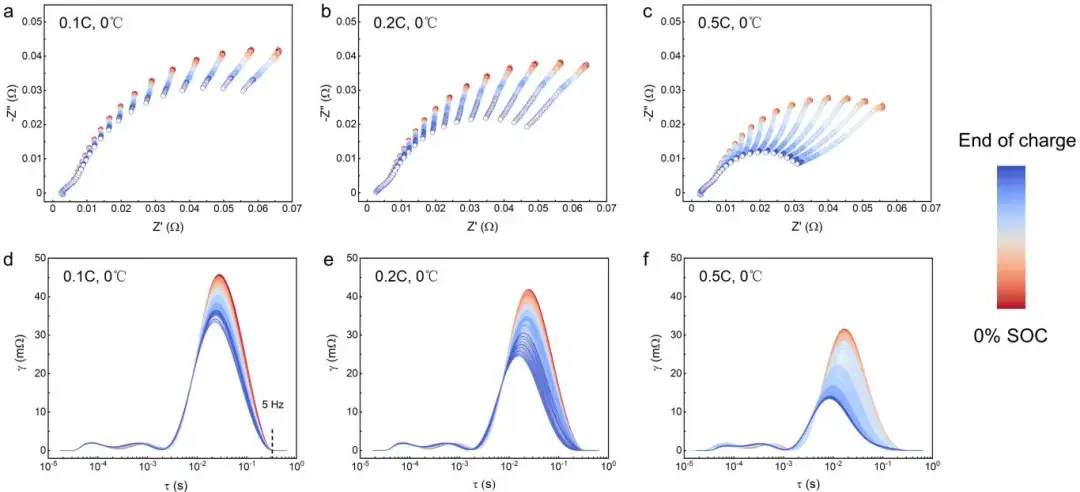
Figure 4. Changes in EIS spectra (Nyquist plot and DRT) of soft-pack batteries during charging at different rates.
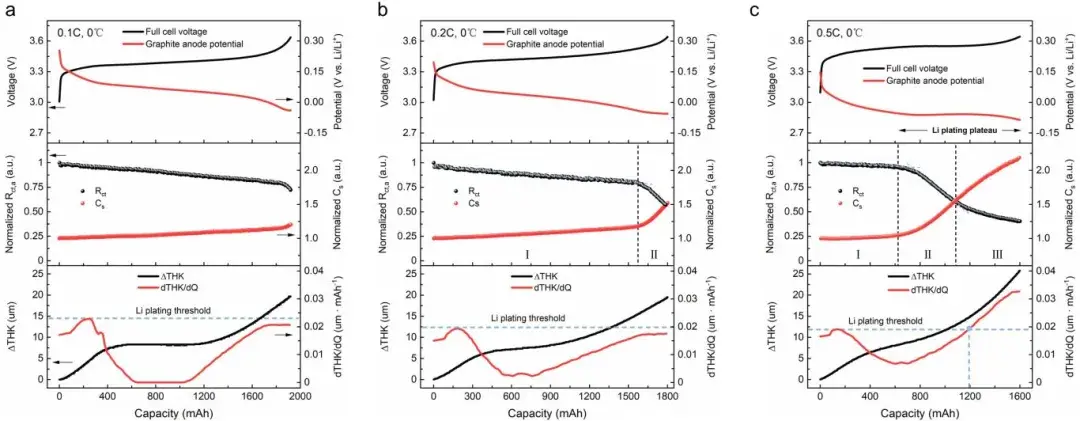
Figure 5. Shows the characteristics of thickness and impedance changes during lithium plating in pouch cells. From top to bottom, it includes the cell voltage curve, negative electrode charge transfer impedance/capacity changes, and thickness variation.
3.2 Thickness metrics (dT/dQ) complement electrochemical sensitivity
-
At low rates (0.1C, 0.2C) and 0°C, dT/dQ remained below the empirically established plating threshold, agreeing with no-plating by DEIS in most cases.
-
At 0.5C and higher SOC, dT/dQ exceeded the threshold after ~1200 mAh of charge at 0°C, providing a macroscopic signature of extensive lithium accumulation (consistent with Stage III).
-
DEIS detected plating onset earlier than thickness methods, whereas dT/dQ better reflected the physical severity and morphology (bulk volume contribution) of deposited lithium.
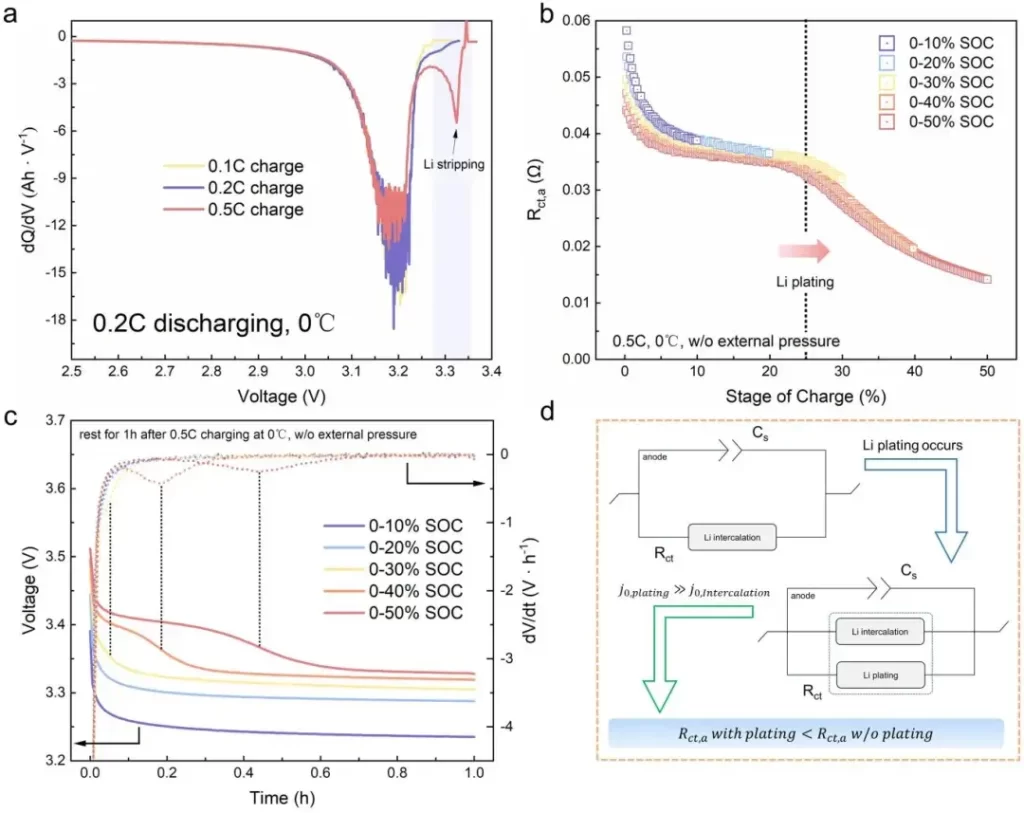
Figure 6. Incremental Capacity Analysis (ICA) and Open Circuit Voltage Relaxation (dOCV) analysis to validate the accuracy of DEIS method in indicating lithium plating.
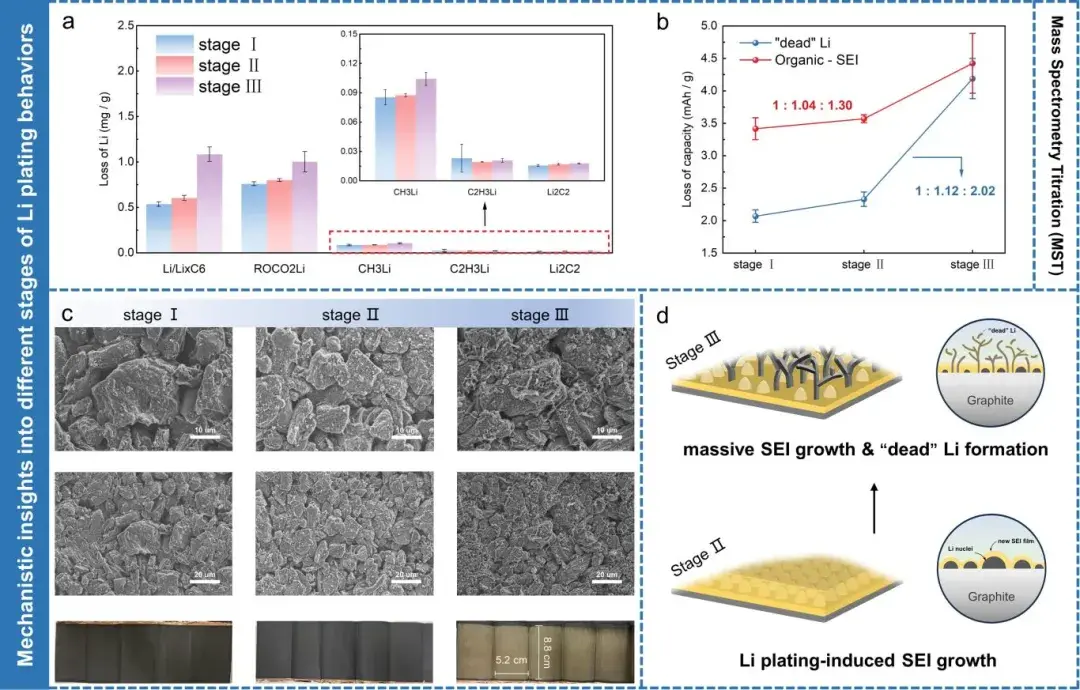
Figure 7. (a) Mass spectrometry titration results of graphite electrode sheets charged to three different stages of Rct,a variation. (b) Capacity loss attributed to “dead lithium” and different organic SEI components. (c) Scanning electron microscopy and optical images of three representative cells at different stages of Rct,a variation. (d) Schematic illustration of lithium plating behavior on graphite surface during Stages II/III of Rct,a variation.
3.3 Ex-situ validation links stages to physical deposits and capacity loss
MST and SEM analyses on cells disassembled at representative Rct,a stages confirmed:
-
Stage I: no detectable metallic Li; normal SEI composition.
-
Stage II: measurable MST-quantified active Li deposits and early SEI organic growth; SEM shows particulate/clustered deposits.
-
Stage III: abundant metallic Li and thick SEI layers; dendritic features observed; significant irreversible capacity loss attributed to dead lithium and SEI formation.
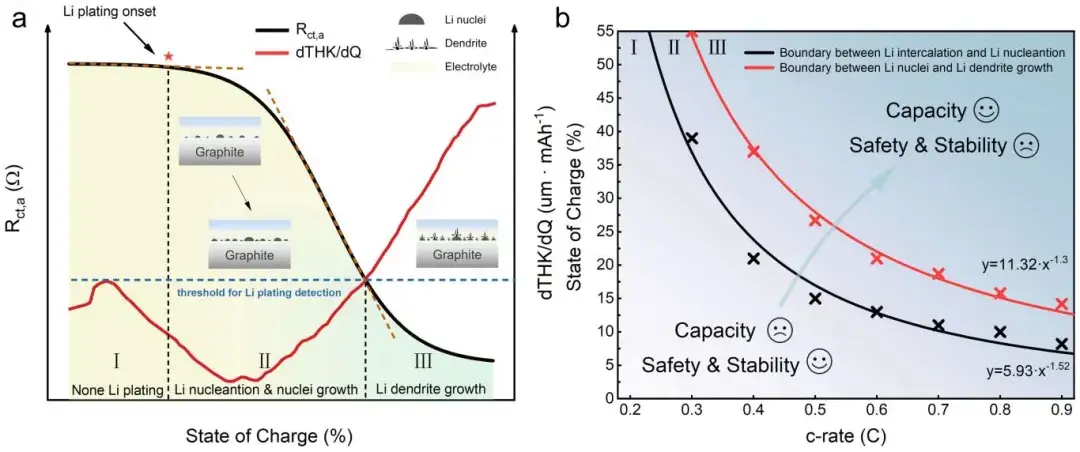
Figure 8. Schematic of in-situ impedance-thickness measurement method monitoring the lithium plating process, along with boundaries indicating different stages of lithium plating occurrence.
During continuous charging cycles, researchers observed that Stage II (lithium nucleation and growth) and Stage III (extensive dendrite growth) in the second cycle occurred earlier compared to the first cycle. They attributed this to residual lithium on the graphite surface from the first discharge cycle, which reduced the nucleation barrier for subsequent Li plating during charging, thereby leading to premature lithium plating. Additionally, researchers cycled three batteries within different SOC ranges corresponding to the proposed stages of Li plating evolution (no plating, lithium nucleation and growth, dendrite growth). They utilized an internally developed Electromotive Force (EMF) measurement method to analyze the State of Health (SOH) and Active Lithium Loss (LLI) of batteries across different cycle numbers. The results indicated that early onset of Li plating and dendrite growth accelerated battery capacity loss, potentially causing capacity drops. This underscores the critical importance of detailed analysis and monitoring of Li plating evolution processes to advance the application of lithium-ion batteries under extreme conditions.
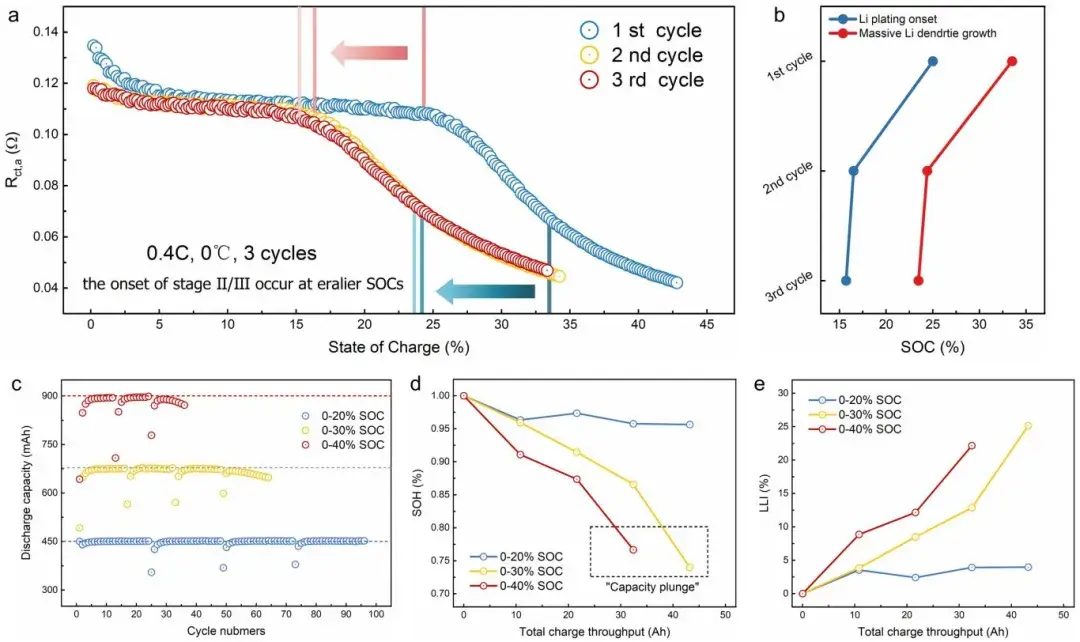
Figure 9. (a) Variation of Rct,a across three cycles, and (b) starting SOC of Stage II/Stage III during these cycles. (c) Discharge capacity variation with cycle number for three batteries cycled within different SOC ranges. Under equivalent total charge throughput, changes in (d) State of Health (SOH) and (e) Active Lithium Loss (LLI) of batteries.
3.4 Cycle-to-cycle advancement of plating
Cells cycled across equivalent charge throughputs showed earlier onset of Stage II/III in subsequent cycles, consistent with residual surface lithium reducing nucleation barriers and accelerating dendrite growth, ultimately accelerating capacity fade.
4. Discussion
4.1 Complementarity of DEIS and thickness monitoring
-
DEIS (Rct,a) is highly sensitive to interfacial electrochemical changes and detects plating initiation earlier, making it valuable for early warning and BMS integration.
-
In-situ thickness (dT/dQ) provides a robust macroscopic measure of deposit growth and morphology that correlates with safety risk and irreversible capacity loss.
Combining these modalities produces multidimensional descriptors (electrochemical + structural) that enable staging of lithium plating and improved prognostics.
4.2 Practical implications for BMS and cell design
-
Early detection: DEIS signatures can serve as precursors for adaptive BMS strategies (dynamic charge current reduction, thermal management).
-
Thresholds and maps: The plotted Rct,a inflection boundaries vs. C-rate and SOC define operational zones (safe lithiation, nucleation risk, dendrite growth) useful for cell-level charge protocols.
-
Aging management: Monitoring plating evolution across cycles informs SOH models and maintenance schedules to limit irreversible LLI.
4. Conclusion
This work demonstrates a robust, in-situ methodology to detect, stage, and quantify lithium plating in commercial pouch C/LiFePO₄ cells by combining DEIS (dynamic impedance) and high-precision thickness monitoring (RSS1400, SEW2100). DEIS reveals a characteristic three-stage Rct,a evolution—(I) lithiation, (II) lithium nucleation/growth, and (III) dendrite growth—while dT/dQ identifies macroscopic deposition and severity aligned to Stage III. Ex-situ MST and SEM confirm physical states corresponding to electrochemical stages. Additionally, without precise monitoring of lithium deposition states, lithium plating can become uncontrollable, potentially causing rapid capacity degradation or even capacity “drop.” This work focuses on analyzing the various evolution processes of lithium plating in commercial pouch cells, providing novel insights into the complex phenomenon of Li plating. It contributes to the development of Battery Management Systems (BMS) and potential applications involving “graphite-lithium” hybrid negative electrodes.
5. Original Article
Ying Lin, Wenxuan Hu, Meifang Ding, Yonggang Hu, Yufan Peng, Jinding Liang, Yimin Wei, Ang Fu, Jianrong Lin, Yong Yang. Unveiling the Three Stages of Li Plating and Dynamic Evolution Processes in Pouch C/LiFePO4 Batteries. Advanced Energy Materials. 2024, 2400894.
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



