-
iestinstrument
In-situ Monitoring of NCM Material Cell Storage and Overcharge Gas Production
1. Preface
Because of its high energy density, NCM materials have become the first choice of cathode materials for most power batteries. The ratio of Ni/Co/Mn has an important influence on the performance of the battery 1-2. As shown in Figure 1, the structure of the nickel-cobalt-manganese NCM material is shown in Figure 6. The increase in Ni content can significantly increase the gram capacity of the material. Ni will cause dislocation mixing with Li, leading to difficulties in de-intercalation of lithium ions, poor electrochemical performance, and poor thermal stability; a certain amount of Co can reduce cation mixing, stabilize the layered structure of the material, and increase the electrical conductivity of the material. However, the more Co content will increase the material cost; the addition of Mn can reduce the material cost and improve the safety and stability of the material. At the same time, if the Mn content is too high, it will easily damage the layered structure, and the spinel phase will appear, which will make the cycle stability poor 3-5.
At present, due to the market’s strong demand for high energy density, NCM materials are developing towards high nickel (even without cobalt). The higher the nickel content, the more alkaline lithium oxides remaining on the surface of the NCM material during synthesis. A large amount of LiOH and Li2CO3, these lithium salts are unstable, and it is easy to produce gas during charge and discharge, high temperature storage and overcharge, which causes the battery to swell and cause safety problems. Therefore, it needs to be coated by doping during the production of NCM materials or other methods to modify it, reduce the risk of NCM material gas production, and further meet the market’s demand for cell safety.
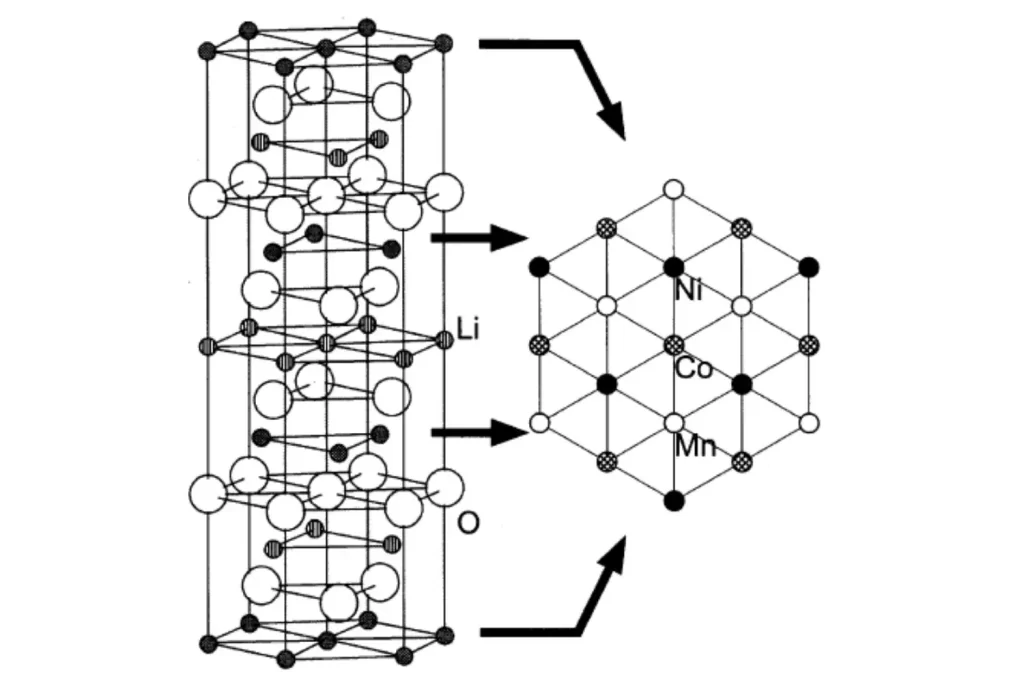
Figure 1. Schematic diagram of the nickel-cobalt-manganese NCM material structure 6
The traditional method usually uses a single volume measurement method, which uses the balance to weigh the cells in different states and convert it into the volume change of the cell as shown in Figure 2(a). This ex-situ method changes the real-time environment and state of the battery cell, which will cause the following problems:
- Single measurement cannot accurately obtain gas production starting point, inflection point and gas production rate.
- The test time lags, it is easy to cause the volume of the battery core to shrink, and the test result is relatively low. As shown in Figure 2(b) and 2(c), after the vented battery core is allowed to stand for 5 minutes, the battery volume has obviously shrunk.
- The balance cannot be placed in a high-temperature environment for measurement, and it is difficult to achieve high-temperature testing of batteries.
- A cell can only get one test point, if you want to obtain process data points, it takes a lot of cells.
- This method has high requirements on the consistency of the battery cells.
This article uses the in-situ gas production volume monitor(GVM Series)to monitor the gas production behavior of the NCM pouch cell under different temperature storage and overcharge conditions to obtain accurate gas production starting points and inflection points, providing a rapid and accurate assessment for R&D The method of material gas production performance.

Figure 2 . (a) Measurement structure diagram of a traditional balance. (b) After the overcharge test is completed, the cell is left for 0min. (c) After the overcharge test is completed, the cell is left for 5min.
2. Gas Production Measurement Principle
Combining Newton’s theorem (equation 1) and Archimedes’ buoyancy law (equation 2), the mass change of the cell during the charging and discharging process is measured in real time by a special sensor, and the cell volume change 3 (equation 3 and 4) is further converted.

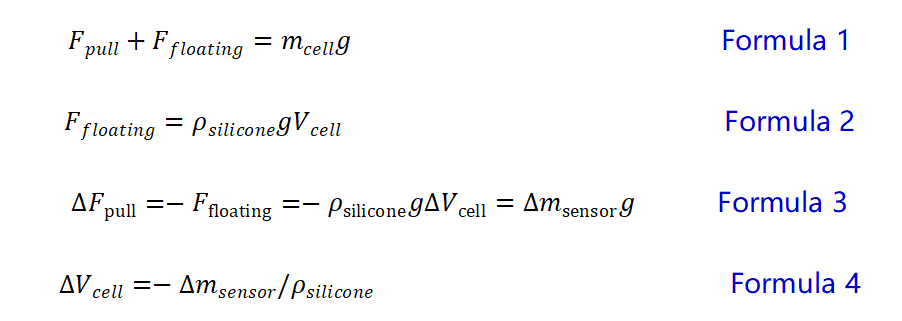
Note: The acceleration of gravity g is 9.8m/s2, and ρ silicone oil is 0.971g/ml (25℃).
3. Experimental Equipment and Test Methods
3.1 Experimental Equipment
Model GVM2200 (IEST), the test temperature range is 20 ℃ ~ 85 ℃, support dual channel (2 cells) synchronous test, the appearance of the device is shown in Figure 3.
Figure 3. Appearance of GVM2200 Device
3.2 Test Method
Initially weigh the cell m0, put the cell to be tested into the corresponding channel of the device, start the MISG software, set the corresponding cell number and sampling frequency parameter of each channel, the software automatically reads the volume change and test temperature , Current, voltage, capacity and other data.
4. In-situ Monitoring of the Gas Production Behavior of the NCM Cell
4.1 Storage Gas Monitoring
In order to further improve the electrochemical performance and storage stability of high-nickel NCM materials, they are usually modified in different ways, such as doping or coating. Figure 4 shows the in-situ gas production monitoring of two 8-series high-nickel NCM materials when they are fully stored at 70°C and 85°C. These two materials are surface-modified on the same NCM substrate: Coat one material (single coated) and coated two materials (double coated). As can be seen from the figure, at 70°C, the total gas production of the two materials is less than 0.4mL, and the volume change percentage is about 6%. The gas production of the single-clad material is slightly larger than that of the double-clad. After 20 minutes of storage, the gas production of both materials began to increase significantly. After 4 hours of storage, the total gas production of the single-clad material reached 2.4 mL, the volume change percentage was about 46%, and the total gas production of the double-clad material was 1.5 mL, volume The change percentage is about 27%, which shows that the double coating method has a significant effect on suppressing the high-temperature storage gas production of the high-nickel material, and the stability of the NCM material can be further improved in this direction in the future. Therefore, using the in-situ method to continuously monitor the storage gas production behavior can obtain the starting point and the maximum point of gas production, which helps the R&D personnel to carry out the next research and development work.
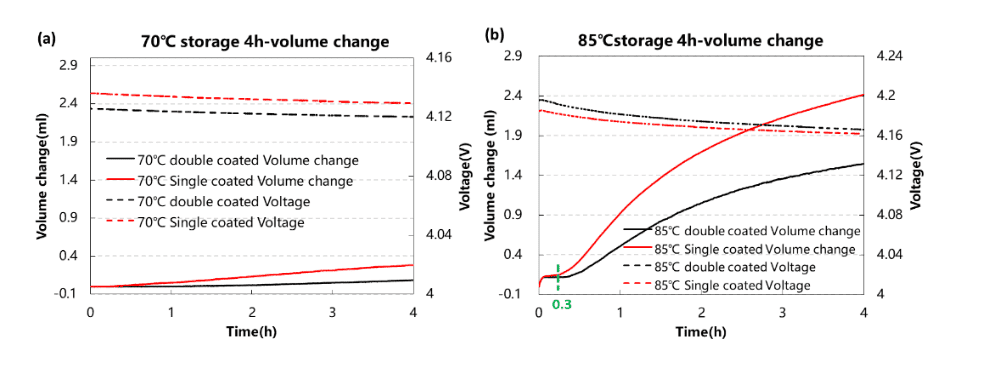
Figure 4. (a) Gas production monitoring at 70℃ full charge storage for 4h. (b) Gas production monitoring at 85℃ full charge storage for 4h.
4.2 Overcharge Gas Production Monitoring
In order to improve the energy density of batteries, high-nickel NCM materials are developing toward high voltage, but under high-voltage conditions, how to maintain the structural stability of high-nickel materials is a major challenge. Monitoring the overcharge gas production behavior of high nickel materials is a characterization method to evaluate the structural stability of materials. As shown in Figure 5, the volume of gas produced by two high-nickel material cells overcharged at 45℃. It can be seen from Figure 5(a) that both materials maintain good structural stability within 100% SOC, and no gas production behavior. Figure 5(b) is an enlarged curve in the range of 100%~120% SOC. High nickel 1 starts gas production at the position of 108%SOC (corresponding voltage of 4.5V), and high nickel 2 starts gas production at the position of 115%SOC (corresponding voltage of 5V or more), which shows that high nickel 2 can keep the structure stable Under the circumstances, adapting to a higher charging voltage and releasing more capacity is more conducive to improving the energy density of the battery cell. Therefore, using the in-situ method to continuously monitor the overcharge gas production behavior can obtain the cell SOC and voltage corresponding to the gas production starting point, which is conducive to the R&D personnel to carry out the next research and development work.
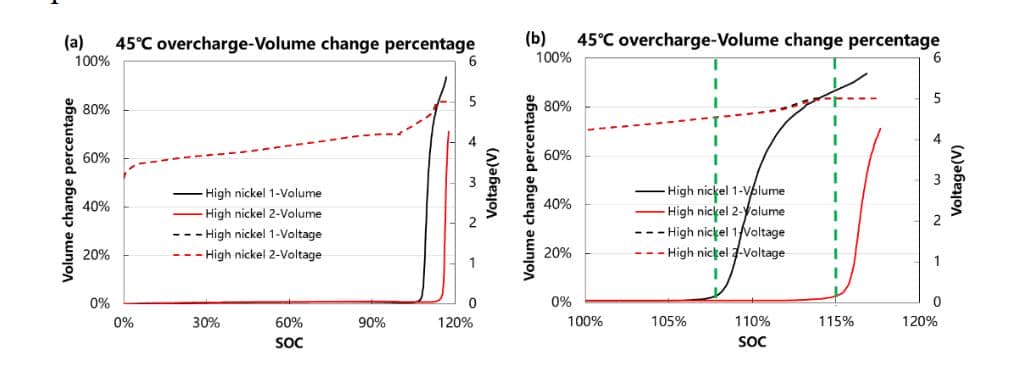
Figure 5. (a) 45℃ overcharge gas production monitoring. (b) 45℃ overcharge gas production enlarged view.
5. Summary
In this paper, a temperature-controlled dual-channel in-situ gas production volume monitor(GVM2200) is used to monitor the gas production behavior of the NCM cell under different temperature storage and overcharge conditions, and the accurate gas production starting point and maximum point, as well as gas production can be obtained. The corresponding cell SOC and voltage at the beginning of the gas provide a method for R&D to quickly and accurately evaluate the gas production performance of the material.
6. References
[1] Brian L. Ellis, Kyu Tae Lee, and Linda F. Nazar. Positive Electrode Materials for Li-Ion and Li-Batteries. Chem. Mater. 22(2010), 691–714 691.
[2] Jing Xie, Yi-Chun Lu. A retrospective on lithium-ion batteries. Nature Communications. (2020) 11:2499.
[3] C. P. Aiken, J. R. Dahn et al. An Apparatus for the Study of In Situ Gas Evolution in Li-Ion Pouch Cells. J. Electro Soc, 161(2014) A1548-A1554.
[4] Yongseon Kim. Mechanism of gas evolution from the cathode of lithium-ion batteries at the initial stage of high-temperature storage. J. Mater Sci, 48(2013), 8547–8551.
[5] Hyung-Joo Noh, Yang-Kook Sun et al. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x=1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources, 233(2013)121-130.
[6] Yukinori Koyamaa, Tsutomu Ohzukub et al. Crystal and electronic structures of superstructural Li1-x[Co1/3Ni1/3Mn1/3]O2 (0 _ x _ 1). J. Power Sources, 119–121(2003) 644–648.
Subscribe Us
Contact Us
If you are interested in our products and want to know more details, please leave a message here, we will reply you as soon as we can.



